Your Treatment ind fda images are ready. Treatment ind fda are a topic that is being searched for and liked by netizens now. You can Get the Treatment ind fda files here. Download all free photos.
If you’re looking for treatment ind fda images information related to the treatment ind fda topic, you have visit the ideal site. Our site frequently provides you with hints for seeing the maximum quality video and picture content, please kindly hunt and find more enlightening video articles and images that fit your interests.
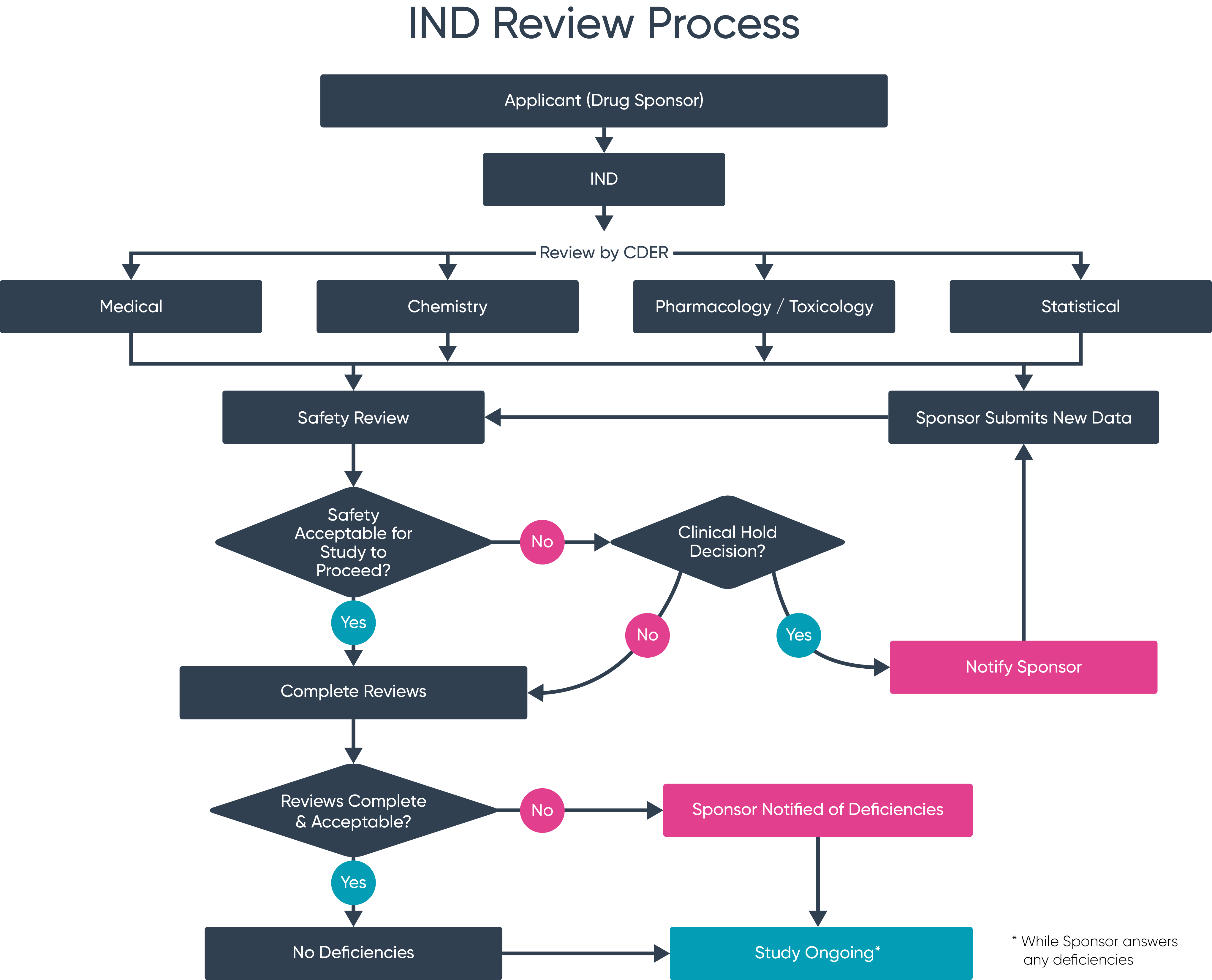
Treatment Ind Fda. Food and Drug Administration FDA clearance of its Investigational New Drug IND application for DCR-AUD the Companys investigational RNAi. As is true for any new IND an access IND goes into effect 30 days after FDA receives the IND or on earlier notification by FDA 21 CFR 31240 and 312305d1. Food and Drug Administration FDA has cleared an Investigational New Drug Application IND that enables the company to proceed with initiating a. Said that the US.
 Translate Bio Announces Fda Clinical Hold On Ind From biospectrumasia.com
Translate Bio Announces Fda Clinical Hold On Ind From biospectrumasia.com
Food and Drug Administration FDA has cleared an Investigational New Drug Application IND that enables the company to proceed with initiating a. This is the first of three phase 2 trials planned to investigate SPI-62 as a treatment for disorders. 29 2021– Dicerna Pharmaceuticals Inc. The FDA accepted the Companys first IND application for NUV-422 in October 2020 for the treatment of patients with high-grade gliomas including glioblastoma multiforme GBM. DRNA Company or Dicerna a leading developer of investigational ribonucleic acid interference RNAi therapeutics today announced the US. PORTLAND OR USA I December 13 2021 ISparrow Pharmaceuticals an emerging clinical-stage biopharmaceutical company developing novel targeted therapies for disorders of corticosteroid excess today announced that the US.
DRNA Company or Dicerna a leading developer of investigational ribonucleic acid interference RNAi therapeutics today announced the US.
DRNA Company or Dicerna a leading developer of investigational ribonucleic acid interference RNAi therapeutics today announced the US. Vitti Labs Announces FDA Approval of IND Application for Phase II Clinical Trial of Combination Mesenchymal Stem Cell and Exosome Treatment of. Food and Drug Administration FDA has cleared an Investigational New Drug Application IND that enables the company to proceed with initiating a phase 2 clinical trial to study SPI-62 a potent and selective investigational HSD-1 inhibitor for the treatment of Cushings disease CD. As is true for any new IND an access IND goes into effect 30 days after FDA receives the IND or on earlier notification by FDA 21 CFR 31240 and 312305d1. Excision Receives FDA Clearance of IND for Phase 12 Trial of EBT-101 CRISPR-Based Therapeutic for Treatment of HIV. The FDA accepted the Companys first IND application for NUV-422 in October 2020 for the treatment of patients with high-grade gliomas including glioblastoma multiforme GBM and.
 Source: complianceonline.com
Source: complianceonline.com
The IND paves the way for us to expeditiously evaluate ketamine and other psychedelics via the FDA regulatory pathway in various mental illness neurological and pain disorders. FDA may ask the sponsor to conduct a clinical study or if the number of patients enrolled increases to submit an IND application for larger population widespread treatment use. The IND paves the way for us to expeditiously evaluate ketamine and other psychedelics via the FDA regulatory pathway in various mental illness neurological and pain disorders. This is the first of three phase 2 trials planned to investigate SPI-62 as a treatment for disorders. Said that the US.
 Source: sitn.hms.harvard.edu
Source: sitn.hms.harvard.edu
Having received clearance for the IND Sparrow plans to begin enrolling patients in early 2022. The FDA accepted the Companys first IND application for NUV-422 in October 2020 for the treatment of patients with high-grade gliomas including glioblastoma multiforme GBM. Having received clearance for the IND Sparrow plans to begin enrolling patients in early 2022. Sparrow Pharmaceuticals an emerging clinical-stage biopharmaceutical company developing novel targeted therapies for disorders of corticosteroid excess today announced that the US. Excision Receives FDA Clearance of IND for Phase 12 Trial of EBT-101 CRISPR-Based Therapeutic for Treatment of HIV.
 Source: biospectrumasia.com
Source: biospectrumasia.com
FDA allowance of the IND application represents a significant milestone for Vasomune and marks the transition of the company to clinical development of a novel investigational medicine targeting the vascular response to SARS-CoV-2 infection for the treatment of seriously ill patients with COVID-19 said Douglas Hamilton President and CEO of Vasomune. PORTLAND Ore December 13 2021–Sparrow Pharmaceuticals an emerging clinical-stage biopharmaceutical company developing novel targeted therapies for disorders of corticosteroid excess today announced that the US. Sparrow Pharmaceuticals Announces FDA Clearance of IND for SPI-62 for the Treatment of Cushings Disease Main Business Finance News Today Broadcom. Excision Receives FDA Clearance of IND for Phase 12 Trial of EBT-101 CRISPR-Based Therapeutic for Treatment of HIV. The IND paves the way for us to expeditiously evaluate ketamine and other psychedelics via the FDA regulatory pathway in various mental illness neurological and pain disorders.
 Source: nccih.nih.gov
Source: nccih.nih.gov
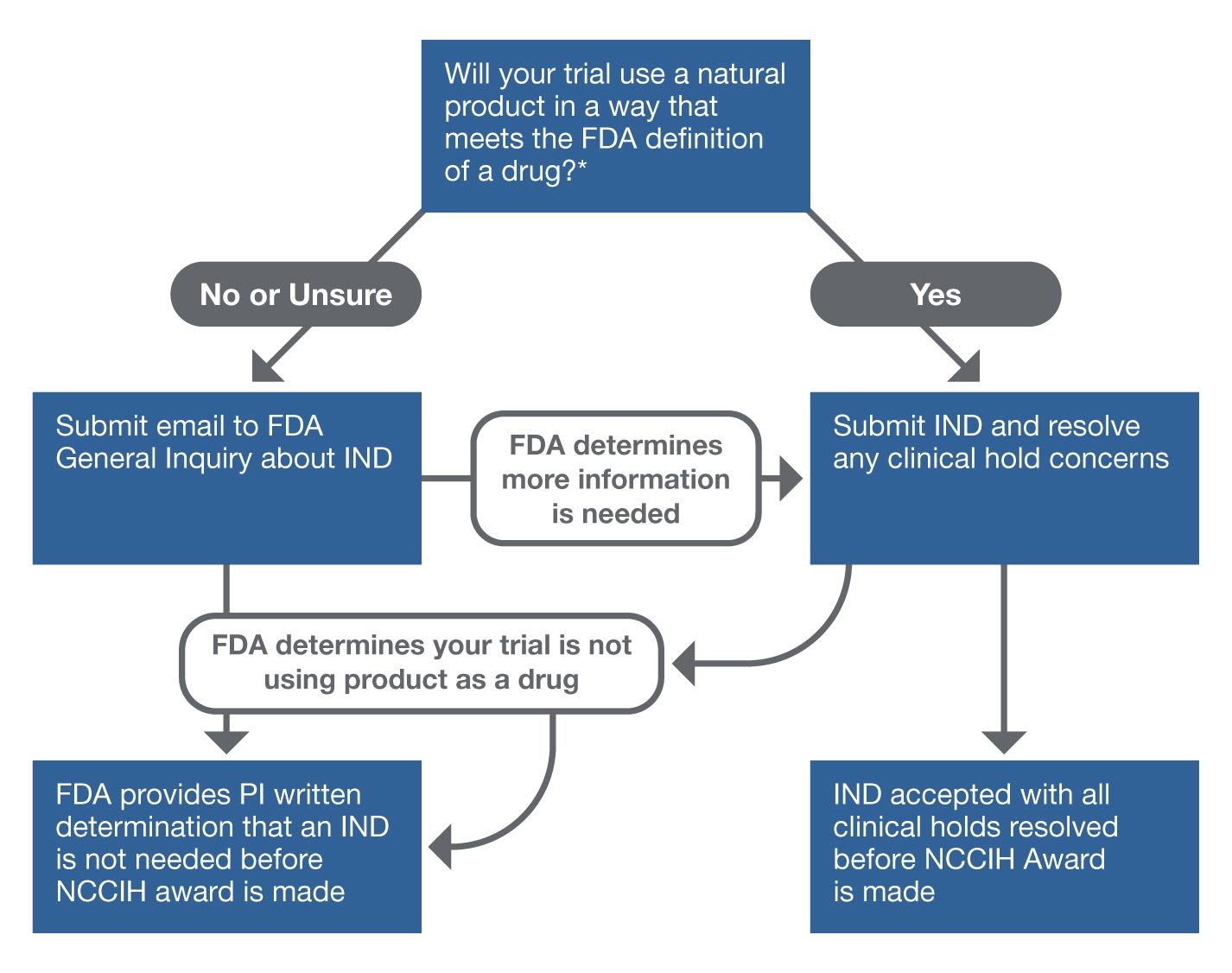
DRNA Company or Dicerna a leading developer of investigational ribonucleic acid interference RNAi therapeutics today announced the US. PORTLAND Ore December 13 2021–Sparrow Pharmaceuticals an emerging clinical-stage biopharmaceutical company developing novel targeted therapies for disorders of corticosteroid excess today announced that the US. For a treatment protocol however access may not begin until 30 days after FDA receives the protocol or on earlier notification by FDA 21 CFR 312305d2ii and IRB approval has been obtained consistent with 21 CFR part 56 see 21. The IND paves the way for us to expeditiously evaluate ketamine and other psychedelics via the FDA regulatory pathway in various mental illness neurological and pain disorders. FDA may ask the sponsor to conduct a clinical study or if the number of patients enrolled increases to submit an IND application for larger population widespread treatment use.
 Source: ideagen.com
Source: ideagen.com
DRNA Company or Dicerna a leading developer of investigational ribonucleic acid interference RNAi therapeutics today announced the US. EBT-101 first-in-human CRISPR-based one. Nuvation Bio Inc. Food and Drug Administration has cleared its investigational new drug application to evaluate NUV-422 a cyclin-dependent kinase CDK 246 inhibitor for the treatment of advanced breast cancerThe FDA accepted the Companys first IND application for NUV-422 in October 2020 for the treatment of patients with high-grade gliomas including. Sparrow Pharmaceuticals an emerging clinical-stage biopharmaceutical company developing novel targeted therapies for disorders of corticosteroid excess today announced that the US.
 Source: genoskin.com
Source: genoskin.com
Food and Drug Administration FDA has cleared an Investigational New Drug Application IND that enables the company to proceed with initiating a phase 2 clinical trial to. 29 2021– Dicerna Pharmaceuticals Inc. For a treatment protocol however access may not begin until 30 days after FDA receives the protocol or on earlier notification by FDA 21 CFR 312305d2ii and IRB approval has been obtained consistent with 21 CFR part 56 see 21. CBMG Receives FDA Clearance of IND Application for Bi-Specific Anti-CD19CD20 CAR-T Cell Therapy for RelapsedRefractory B-cell Non-Hodgkin Lymphoma By. Excision Receives FDA Clearance of IND for Phase 12 Trial of EBT-101 CRISPR-Based Therapeutic for Treatment of HIV.
 Source: drug-dev.com
Source: drug-dev.com
Fabio Chianelli Chief Executive Officer of PharmaTher said The FDAs acceptance of our IND application for ketamine to treat Parkinsons disease is a significant milestone for us. Nuvation Bio Inc. Food and Drug Administration FDA has cleared an Investigational New Drug Application IND that enables the company to proceed with initiating a phase 2 clinical trial to study SPI-62 a potent and selective. Said that the US. Having received clearance for the IND Sparrow plans to begin enrolling patients in early 2022.
 Source: researchgate.net
Source: researchgate.net
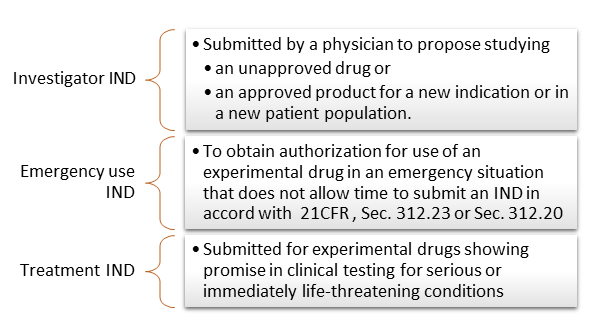
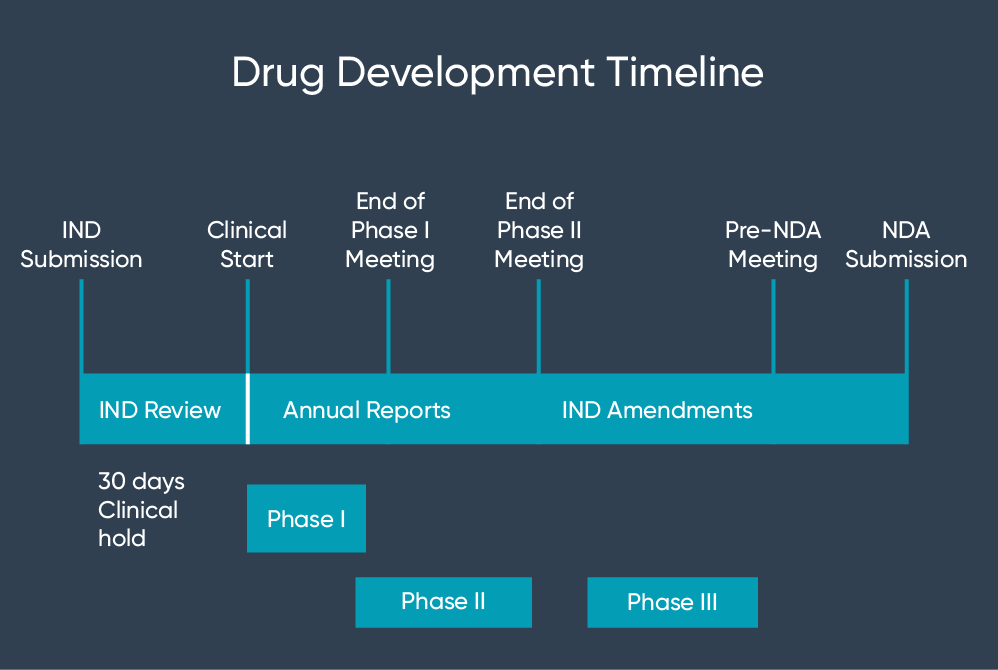
The treatment IND 21 CFR 31234 and 31235 is a mechanism for providing eligible subjects with investigational drugs for the treatment of serious and life-threatening illnesses. Having received clearance for the IND Sparrow plans to begin enrolling patients in early 2022. CBMG Receives FDA Clearance of IND Application for Bi-Specific Anti-CD19CD20 CAR-T Cell Therapy for RelapsedRefractory B-cell Non-Hodgkin Lymphoma By. Fabio Chianelli Chief Executive Officer of PharmaTher said The FDAs acceptance of our IND application for ketamine to treat Parkinsons disease is a significant milestone for us. Food and Drug Administration FDA has cleared an Investigational New Drug Application IND that enables the company to proceed with initiating a phase 2 clinical trial to study SPI-62 a potent and selective investigational HSD-1 inhibitor for the treatment of Cushings disease CD.
 Source: policymed.com
Source: policymed.com
CBMG Receives FDA Clearance of IND Application for Bi-Specific Anti-CD19CD20 CAR-T Cell Therapy for RelapsedRefractory B-cell Non-Hodgkin Lymphoma By. In addition for antimicrobial products the FDA has a consultation program to facilitate communications between the sponsor and the FDA before filing an IND involving the treatment of bacterial fungal and viral infections opportunistic infections emerging infections including naturally emerging diseases and potential biothreat agents topical microbicides directed at prevention of HIV transmission and. Food and Drug Administration FDA has cleared an Investigational New Drug Application IND that enables the company to proceed with initiating a phase 2 clinical trial to study SPI-62 a potent and selective. The treatment IND 21 CFR 31234 and 31235 is a mechanism for providing eligible subjects with investigational drugs for the treatment of serious and life-threatening illnesses. Food and Drug Administration FDA clearance of its Investigational New Drug IND application for DCR-AUD the Companys investigational RNAi.
 Source: irb.utah.edu
Source: irb.utah.edu
Sparrow Pharmaceuticals today announced that the US. As is true for any new IND an access IND goes into effect 30 days after FDA receives the IND or on earlier notification by FDA 21 CFR 31240 and 312305d1. FDA allowance of the IND application represents a significant milestone for Vasomune and marks the transition of the company to clinical development of a novel investigational medicine targeting the vascular response to SARS-CoV-2 infection for the treatment of seriously ill patients with COVID-19 said Douglas Hamilton President and CEO of Vasomune. Sparrow Pharmaceuticals an emerging clinical-stage biopharmaceutical company developing novel targeted therapies for disorders of corticosteroid excess today announced that the US. Food and Drug Administration FDA has cleared an Investigational New Drug Application IND that enables the company to proceed with initiating a phase 2 clinical trial to study SPI-62 a potent and selective.
 Source: regardd.org
Source: regardd.org
The FDA accepted the Companys first IND application for NUV-422 in October 2020 for the treatment of patients with high-grade gliomas including glioblastoma multiforme GBM. Sparrow Pharmaceuticals an emerging clinical-stage biopharmaceutical company developing novel targeted therapies for disorders of corticosteroid excess today announced that the US. DRNA Company or Dicerna a leading developer of investigational ribonucleic acid interference RNAi therapeutics today announced the US. As is true for any new IND an access IND goes into effect 30 days after FDA receives the IND or on earlier notification by FDA 21 CFR 31240 and 312305d1. PORTLAND Ore December 13 2021–Sparrow Pharmaceuticals an emerging clinical-stage biopharmaceutical company developing novel targeted therapies for disorders of corticosteroid excess today announced that the US.
 Source: ideagen.com
Source: ideagen.com
Sparrow Pharmaceuticals Announces FDA Clearance of IND for SPI-62 for the Treatment of Cushings Disease Main Business Finance News Today Broadcom. The treatment IND 21 CFR 31234 and 31235 is a mechanism for providing eligible subjects with investigational drugs for the treatment of serious and life-threatening illnesses. Food and Drug Administration FDA has cleared an Investigational New Drug Application IND that enables the company to proceed with initiating a phase 2 clinical trial to study SPI-62 a potent and selective. Food and Drug Administration FDA clearance of its Investigational New Drug IND application for DCR-AUD the Companys investigational RNAi. Nuvation Bio Inc.
 Source: ideagen.com
Source: ideagen.com
29 2021– Dicerna Pharmaceuticals Inc. FDA allowance of the IND application represents a significant milestone for Vasomune and marks the transition of the company to clinical development of a novel investigational medicine targeting the vascular response to SARS-CoV-2 infection for the treatment of seriously ill patients with COVID-19 said Douglas Hamilton President and CEO of Vasomune. The FDA accepted the Companys first IND application for NUV-422 in October 2020 for the treatment of patients with high-grade gliomas including glioblastoma multiforme GBM and. Fabio Chianelli Chief Executive Officer of PharmaTher said The FDAs acceptance of our IND application for ketamine to treat Parkinsons disease is a significant milestone for us. Sparrow Pharmaceuticals an emerging clinical-stage biopharmaceutical company developing novel targeted therapies for disorders of corticosteroid excess today announced that the US.
 Source: researchgate.net
Source: researchgate.net
FDA may ask the sponsor to conduct a clinical study or if the number of patients enrolled increases to submit an IND application for larger population widespread treatment use. EBT-101 first-in-human CRISPR-based one. As is true for any new IND an access IND goes into effect 30 days after FDA receives the IND or on earlier notification by FDA 21 CFR 31240 and 312305d1. This is the first of three phase 2 trials planned to investigate SPI-62 as a treatment for disorders. Food and Drug Administration FDA has cleared an Investigational New Drug Application IND that enables the company to proceed with initiating a.

The IND paves the way for us to expeditiously evaluate ketamine and other psychedelics via the FDA regulatory pathway in various mental illness neurological and pain disorders. The FDA accepted the Companys first IND application for NUV-422 in October 2020 for the treatment of patients with high-grade gliomas including glioblastoma multiforme GBM and. In addition for antimicrobial products the FDA has a consultation program to facilitate communications between the sponsor and the FDA before filing an IND involving the treatment of bacterial fungal and viral infections opportunistic infections emerging infections including naturally emerging diseases and potential biothreat agents topical microbicides directed at prevention of HIV transmission and. Excision Receives FDA Clearance of IND for Phase 12 Trial of EBT-101 CRISPR-Based Therapeutic for Treatment of HIV. The IND paves the way for us to expeditiously evaluate ketamine and other psychedelics via the FDA regulatory pathway in various mental illness neurological and pain disorders.
 Source: impactpharma.com
Source: impactpharma.com
The FDA IND is our first of many we will aim to obtain and we are one of the few psychedelics-focused biotech companies that have an IND approved by the FDA for a recognized psychedelic drug. CBMG Receives FDA Clearance of IND Application for Bi-Specific Anti-CD19CD20 CAR-T Cell Therapy for RelapsedRefractory B-cell Non-Hodgkin Lymphoma By. Said that the US. Food and Drug Administration FDA clearance of its Investigational New Drug IND application for DCR-AUD the Companys investigational RNAi. Sparrow Pharmaceuticals an emerging clinical-stage biopharmaceutical company developing novel targeted therapies for disorders of corticosteroid excess today announced that the US.
 Source: ideagen.com
Source: ideagen.com
In addition for antimicrobial products the FDA has a consultation program to facilitate communications between the sponsor and the FDA before filing an IND involving the treatment of bacterial fungal and viral infections opportunistic infections emerging infections including naturally emerging diseases and potential biothreat agents topical microbicides directed at prevention of HIV transmission and. CBMG Receives FDA Clearance of IND Application for Bi-Specific Anti-CD19CD20 CAR-T Cell Therapy for RelapsedRefractory B-cell Non-Hodgkin Lymphoma By. Food and Drug Administration has cleared its investigational new drug application to evaluate NUV-422 a cyclin-dependent kinase CDK 246 inhibitor for the treatment of advanced breast cancerThe FDA accepted the Companys first IND application for NUV-422 in October 2020 for the treatment of patients with high-grade gliomas including. Food and Drug Administration FDA clearance of its Investigational New Drug IND application for DCR-AUD the Companys investigational RNAi. Food and Drug Administration FDA has cleared an Investigational New Drug Application IND that enables the company to proceed with initiating a phase 2 clinical trial to.
 Source: drugtopics.com
Source: drugtopics.com
PORTLAND Ore December 13 2021–Sparrow Pharmaceuticals an emerging clinical-stage biopharmaceutical company developing novel targeted therapies for disorders of corticosteroid excess today announced that the US. PORTLAND OR USA I December 13 2021 ISparrow Pharmaceuticals an emerging clinical-stage biopharmaceutical company developing novel targeted therapies for disorders of corticosteroid excess today announced that the US. Having received clearance for the IND Sparrow plans to begin enrolling patients in early 2022. Said that the US. In addition for antimicrobial products the FDA has a consultation program to facilitate communications between the sponsor and the FDA before filing an IND involving the treatment of bacterial fungal and viral infections opportunistic infections emerging infections including naturally emerging diseases and potential biothreat agents topical microbicides directed at prevention of HIV transmission and.
This site is an open community for users to share their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site good, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title treatment ind fda by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.





