Your Treatment for covid 19 mono images are ready in this website. Treatment for covid 19 mono are a topic that is being searched for and liked by netizens now. You can Get the Treatment for covid 19 mono files here. Get all royalty-free vectors.
If you’re looking for treatment for covid 19 mono pictures information connected with to the treatment for covid 19 mono interest, you have visit the right blog. Our site frequently gives you suggestions for downloading the maximum quality video and picture content, please kindly surf and find more enlightening video content and images that fit your interests.
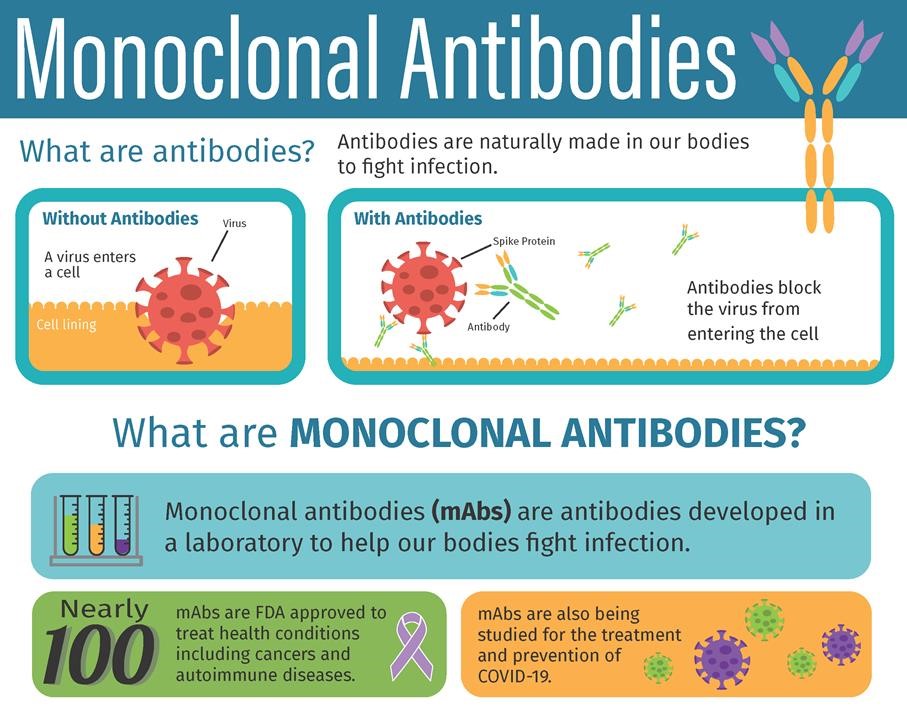
Treatment For Covid 19 Mono. The vast majority are called monoclonal antibodies. Treatment is not authorized by the FDA EUA. Monoclonal antibodies mAbs that can bind to and neutralize the virus in infected patients are a novel class of antiviral intervention 1 2. While vaccines provide the best protection from COVID-19 treatment options such as monoclonal antibodies are available if you have had symptoms of COVID-19 for 10 days or less or have been exposed to COVID-19.
 What Is The Best Way To Treat Covid 19 Remdesivir And Plasma Are Promising But Other Drugs Are Needed From nbcnews.com
What Is The Best Way To Treat Covid 19 Remdesivir And Plasma Are Promising But Other Drugs Are Needed From nbcnews.com
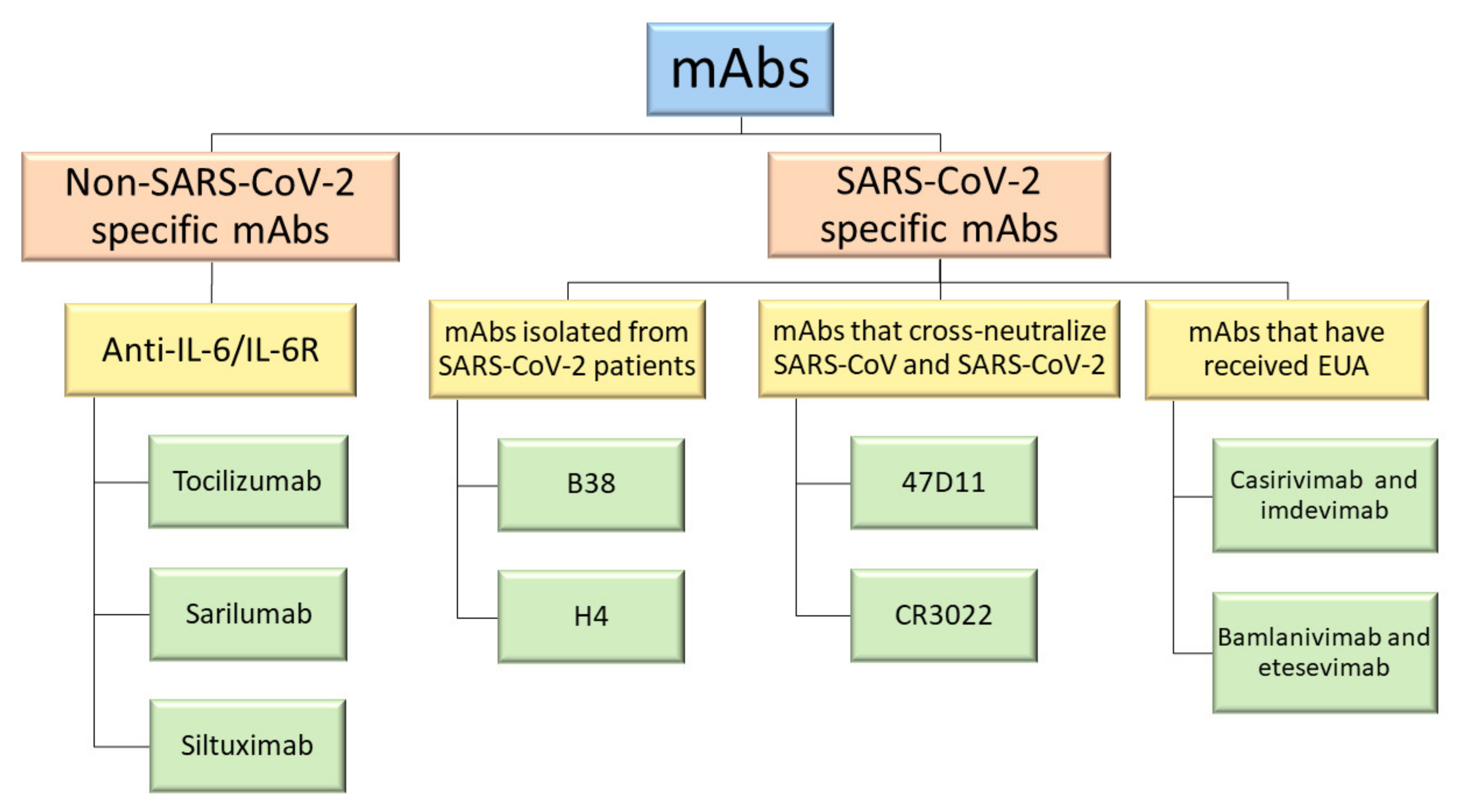
REGEN-COV commonly referred to antibody treatment or monoclonal antibody treatment is a medicine used to treat mild to moderate symptoms of COVID-19 in non-hospitalized adults and children 12 years of age and older weighing at least 88 pounds who are at high risk of developing severe COVID-19 symptoms or the need for hospitalization. She emailed her rheumatologist and pulmonologist and researched the only effective treatments for early COVID-19. If taken early they can reduce the risk of severe disease hospitalization and death. MABs currently approved for emergency use are. At the same time Upinder Singh MD the Stanford Medicine division chief of infectious disease as well as a professor of medicine and of microbiology and immunology was conducting her own investigation. They are administered for the treatment of mild to moderate COVID-19 in adults and pediatric patients 12 years of age or older and who are at high risk for progressing to severe COVID-19.
IVIG is one of the treatments that scientists and doctors are looking to utilize to treat deteriorating patients with COVID-19.
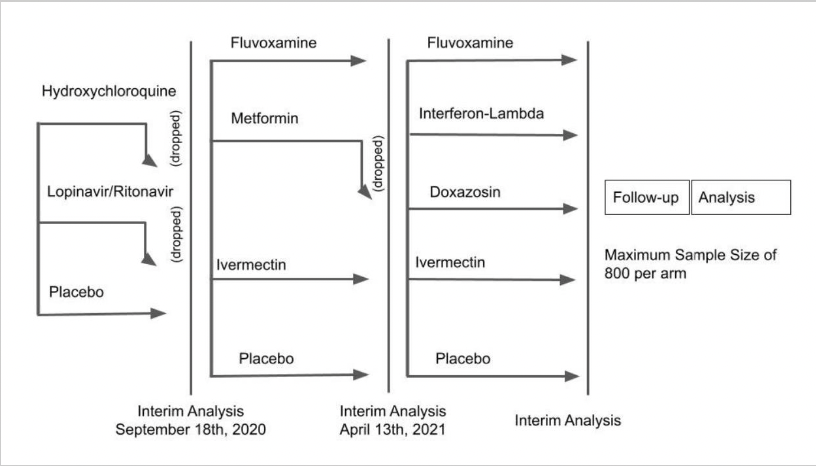
Monoclonal antibody therapy is a way of treating COVID-19 for people who have tested positive have had mild symptoms for seven days or less and are at high risk for developing more serious symptoms. At the same time Upinder Singh MD the Stanford Medicine division chief of infectious disease as well as a professor of medicine and of microbiology and immunology was conducting her own investigation. The dosing is the same for both indications casirivimab 600mg and imdevimab 600mg. Hospitalized due to COVID-19 Require oxygen therapy due to COVID-19 OR Require an increase in baseline oxygen flow rate due to COVID-19 in those on chronic oxygen therapy due to underlying non-COVID-19 related comorbidity. If taken early they can reduce the risk of severe disease hospitalization and death. For use in patients with any of the following conditions.
 Source: iavi.org
Source: iavi.org
REGEN-COV commonly referred to antibody treatment or monoclonal antibody treatment is a medicine used to treat mild to moderate symptoms of COVID-19 in non-hospitalized adults and children 12 years of age and older weighing at least 88 pounds who are at high risk of developing severe COVID-19 symptoms or the need for hospitalization. The FDA has authorized monoclonal antibody treatment for emergency use to treat high-risk patients who test positive and to prevent COVID-19 in a high-risk person whos been exposed. The vast majority are called monoclonal antibodies. At this time we are treating immunocompromised patients with monoclonal antibodies who may still be vulnerable to COVID-19 even if they are fully vaccinated explained Cohen. The virus or the immune system.
 Source: houstonmethodist.org
Source: houstonmethodist.org
If taken early they can reduce the risk of severe disease hospitalization and death. At this time UCHealth uses these products which are available by FDA Emergency Use Authorization. Bamlanivimab and etesevimab administered together. At the same time Upinder Singh MD the Stanford Medicine division chief of infectious disease as well as a professor of medicine and of microbiology and immunology was conducting her own investigation. The virus or the immune system.
 Source: deaconess.com
Source: deaconess.com
Treatment with COVID-19 monoclonal antibodies is done through a one-time intravenous IV infusion or by subcutaneous injection shot given under the skin. Their goal is to confer immunity called passive immunity to the hospitalized COVID-19 patients by taking the antibody containing plasma from recovered patients. For use in patients with any of the following conditions. If you have been diagnosed with COVID-19 and are at high risk for developing severe COVID-19 you may be eligible for monoclonal antibody treatment which might prevent you from becoming sicker. The FDA has authorized monoclonal antibody treatment for emergency use to treat high-risk patients who test positive and to prevent COVID-19 in a high-risk person whos been exposed.
 Source: verywellhealth.com
Source: verywellhealth.com
The goal of this therapy is to help prevent hospitalizations reduce viral loads and lessen symptom severity. Monoclonal antibody therapy is a way of treating COVID-19 for people who have tested positive have had mild symptoms for seven days or less and are at high risk for developing more serious symptoms. IVIG is one of the treatments that scientists and doctors are looking to utilize to treat deteriorating patients with COVID-19. They are administered for the treatment of mild to moderate COVID-19 in adults and pediatric patients 12 years of age or older and who are at high risk for progressing to severe COVID-19. REGEN-COV commonly referred to antibody treatment or monoclonal antibody treatment is a medicine used to treat mild to moderate symptoms of COVID-19 in non-hospitalized adults and children 12 years of age and older weighing at least 88 pounds who are at high risk of developing severe COVID-19 symptoms or the need for hospitalization.
 Source: healthaffairs.org
Source: healthaffairs.org
The FDA has also authorized the monoclonal antibody treatment Actemra tocilizumab to treat COVID-19 in hospitalized adults and those aged two and up who are receiving systemic corticosteroids. She emailed her rheumatologist and pulmonologist and researched the only effective treatments for early COVID-19. Today the FDA issued an emergency use authorization for the investigational monoclonal antibody therapy bamlanivimab for the treatment of mild-to-moderate COVID-19 in adult and pediatric patients. In the midst of the current COVID-19 pandemic a variety of prophylactic and therapeutic treatments are being developed or repurposed to combat COVID-19. The Medicines and Healthcare products Regulatory Agency MHRA has today given approval for the first monoclonal antibody treatment for the.
 Source: blogs.jwatch.org
Source: blogs.jwatch.org
The media host says he used ivermectin a medication that the FDA has warned against. Government is currently supplying REGEN-COV casirivimab and imdevimab for the treatment and post-exposure prophylaxis of COVID-19. Bamlanivimab and etesevimab administered together. The FDA has authorized monoclonal antibody treatment for emergency use to treat high-risk patients who test positive and to prevent COVID-19 in a high-risk person whos been exposed. The virus or the immune system.
 Source: healthcare.utah.edu
Source: healthcare.utah.edu
The virus or the immune system. Monoclonal antibodies are used to neutralize the COVID-19 virus and intended to prevent progression of disease. Treatment is not authorized by the FDA EUA. The FDA has also authorized the monoclonal antibody treatment Actemra tocilizumab to treat COVID-19 in hospitalized adults and those aged two and up who are receiving systemic corticosteroids. Sotrovimab EUA issued May 26 2021 Tocilizumab EUA issued June 24 2021 The FDA authorized the use of these monoclonal antibody therapies to treat mild-to-moderate COVID-19 in adults and pediatric patients when both of these apply.
 Source: mdpi.com
Source: mdpi.com
Monoclonal antibody therapy is a way of treating COVID-19 for people who have tested positive have had mild symptoms for seven days or less and are at high risk for developing more serious symptoms. MABs currently approved for emergency use are. The FDA has also authorized the monoclonal antibody treatment Actemra tocilizumab to treat COVID-19 in hospitalized adults and those aged two and up who are receiving systemic corticosteroids. The FDA has authorized monoclonal antibody treatment for emergency use to treat high-risk patients who test positive and to prevent COVID-19 in a high-risk person whos been exposed. The vast majority are called monoclonal antibodies.
 Source: npr.org
Source: npr.org
Treatment with COVID-19 monoclonal antibodies is done through a one-time intravenous IV infusion or by subcutaneous injection shot given under the skin. Treatment with COVID-19 monoclonal antibodies is done through a one-time intravenous IV infusion or by subcutaneous injection shot given under the skin. Monoclonal antibodies mAbs that can bind to and neutralize the virus in infected patients are a novel class of antiviral intervention 1 2. For use in patients with any of the following conditions. At the same time Upinder Singh MD the Stanford Medicine division chief of infectious disease as well as a professor of medicine and of microbiology and immunology was conducting her own investigation.
 Source: sciencedirect.com
Source: sciencedirect.com
9 2020 the FDA issued an EUA for a single infusion of 700 mg bamlanivimab for the treatment of mild-to-moderate COVID-19 in adult and certain pediatric patients. The vast majority are called monoclonal antibodies. If you have been diagnosed with COVID-19 and are at high risk for developing severe COVID-19 you may be eligible for monoclonal antibody treatment which might prevent you from becoming sicker. REGEN-COV commonly referred to antibody treatment or monoclonal antibody treatment is a medicine used to treat mild to moderate symptoms of COVID-19 in non-hospitalized adults and children 12 years of age and older weighing at least 88 pounds who are at high risk of developing severe COVID-19 symptoms or the need for hospitalization. What Are Monoclonal Antibodies.
 Source: npr.org
Source: npr.org
9 2020 the FDA issued an EUA for a single infusion of 700 mg bamlanivimab for the treatment of mild-to-moderate COVID-19 in adult and certain pediatric patients. This type of drug is not new and many are currently approved to treat conditions such as cancer or rheumatoid arthritis. MABs currently approved for emergency use are. While vaccines provide the best protection from COVID-19 treatment options such as monoclonal antibodies are available if you have had symptoms of COVID-19 for 10 days or less or have been exposed to COVID-19. There are different treatment options for patients who are hospitalized or require oxygen therapy due to COVID-19.
 Source: thelancet.com
Source: thelancet.com
What Are Monoclonal Antibodies. What Are Monoclonal Antibodies. If you have been diagnosed with COVID-19 and are at high risk for developing severe COVID-19 you may be eligible for monoclonal antibody treatment which might prevent you from becoming sicker. Monoclonal antibodies are used to neutralize the COVID-19 virus and intended to prevent progression of disease. The media host says he used ivermectin a medication that the FDA has warned against.
 Source: menarini.com
Source: menarini.com
9 2020 the FDA issued an EUA for a single infusion of 700 mg bamlanivimab for the treatment of mild-to-moderate COVID-19 in adult and certain pediatric patients. Monoclonal antibodies are used to neutralize the COVID-19 virus and intended to prevent progression of disease. Government is currently supplying REGEN-COV casirivimab and imdevimab for the treatment and post-exposure prophylaxis of COVID-19. Their goal is to confer immunity called passive immunity to the hospitalized COVID-19 patients by taking the antibody containing plasma from recovered patients. At this time UCHealth uses these products which are available by FDA Emergency Use Authorization.

While vaccines provide the best protection from COVID-19 treatment options such as monoclonal antibodies are available if you have had symptoms of COVID-19 for 10 days or less or have been exposed to COVID-19. The FDA has authorized monoclonal antibody treatment for emergency use to treat high-risk patients who test positive and to prevent COVID-19 in a high-risk person whos been exposed. The vast majority are called monoclonal antibodies. If you have been diagnosed with COVID-19 and are at high risk for developing severe COVID-19 you may be eligible for monoclonal antibody treatment which might prevent you from becoming sicker. At the same time Upinder Singh MD the Stanford Medicine division chief of infectious disease as well as a professor of medicine and of microbiology and immunology was conducting her own investigation.
 Source: frontiersin.org
Source: frontiersin.org
Treatment with COVID-19 monoclonal antibodies is done through a one-time intravenous IV infusion or by subcutaneous injection shot given under the skin. The Medicines and Healthcare products Regulatory Agency MHRA has today given approval for the first monoclonal antibody treatment for the. For use in patients with any of the following conditions. The media host says he used ivermectin a medication that the FDA has warned against. Treatment is not authorized by the FDA EUA.
 Source: oatext.com
Source: oatext.com
Government is currently supplying REGEN-COV casirivimab and imdevimab for the treatment and post-exposure prophylaxis of COVID-19. Treatment is not authorized by the FDA EUA. Monoclonal antibodies are used to neutralize the COVID-19 virus and intended to prevent progression of disease. For use in patients with any of the following conditions. The dosing is the same for both indications casirivimab 600mg and imdevimab 600mg.
 Source: nbcnews.com
Source: nbcnews.com
This type of drug is not new and many are currently approved to treat conditions such as cancer or rheumatoid arthritis. Treatment is not authorized by the FDA EUA. The patient has a positive COVID-19 test result. Government is currently supplying REGEN-COV casirivimab and imdevimab for the treatment and post-exposure prophylaxis of COVID-19. The Medicines and Healthcare products Regulatory Agency MHRA has today given approval for the first monoclonal antibody treatment for the.
 Source: ce.mayo.edu
Source: ce.mayo.edu
If you have been diagnosed with COVID-19 and are at high risk for developing severe COVID-19 you may be eligible for monoclonal antibody treatment which might prevent you from becoming sicker. Hospitalized due to COVID-19 Require oxygen therapy due to COVID-19 OR Require an increase in baseline oxygen flow rate due to COVID-19 in those on chronic oxygen therapy due to underlying non-COVID-19 related comorbidity. For use in patients with any of the following conditions. The vast majority are called monoclonal antibodies. In the midst of the current COVID-19 pandemic a variety of prophylactic and therapeutic treatments are being developed or repurposed to combat COVID-19.
This site is an open community for users to do submittion their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site beneficial, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title treatment for covid 19 mono by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.





