Your Treatment for covid 19 infusion images are ready. Treatment for covid 19 infusion are a topic that is being searched for and liked by netizens now. You can Download the Treatment for covid 19 infusion files here. Download all free photos.
If you’re looking for treatment for covid 19 infusion pictures information related to the treatment for covid 19 infusion keyword, you have come to the right blog. Our site always provides you with suggestions for viewing the highest quality video and image content, please kindly surf and locate more enlightening video articles and images that match your interests.
Treatment For Covid 19 Infusion. It contains man-made antibodies similar to the antibodies of people who have recovered from COVID. Monoclonal antibody treatment is not authorized for use in patients who are. Monoclonal antibody mAb therapy also called monoclonal antibody infusion treatment is a way of treating COVID-19. COVID-19 Monoclonal Antibody Therapy Infusion For Patients If you have recently been diagnosed with COVID-19 there may be treatment options available to you.
 Mesenchymal Stem Cell Therapy For Severe Covid 19 Signal Transduction And Targeted Therapy From nature.com
Mesenchymal Stem Cell Therapy For Severe Covid 19 Signal Transduction And Targeted Therapy From nature.com
MedStar Health is offering a treatment known as monoclonal antibody infusion to help patients who have been diagnosed with COVID-19 and are at risk of developing a severe case of the illness. Some COVID-19 patients will be able to receive an antibody infusion treatment from U of L Health that is designed to help the body fight off the virus. Health care providers and scientists are investigating other drugs and treatments that may slow or reduce the virus. Patients must be referred by a Medical City Healthcare physician. It contains man-made antibodies similar to the antibodies of people who have recovered from COVID. Clinical trials show that Regenerons monoclonal antibody treatment a combination of two antibodies called casirivimab and imdevimab reduces COVID-19-related hospitalization or deaths in high.
What is Monoclonal Antibody Infusion Therapy.
This type of therapy relies on monoclonal antibodies. MedStar Health is offering a treatment known as monoclonal antibody infusion to help patients who have been diagnosed with COVID-19 and are at risk of developing a severe case of the illness. Therefore high-dose vitamin C infusion may be a significantly effective therapeutic agent in COVID-19 treatment. Thats important because of the variations of the virus that can occur as well as the fact that some fully vaccinated persons may still experience bad symptoms if they catch COVID-19. Health care providers and scientists are investigating other drugs and treatments that may slow or reduce the virus. Some COVID-19 patients will be able to receive an antibody infusion treatment from U of L Health that is designed to help the body fight off the virus.
 Source: tmcaz.com
Source: tmcaz.com
COVID-19 Monoclonal Antibody Therapy Infusion For Patients If you have recently been diagnosed with COVID-19 there may be treatment options available to you. Therefore high-dose vitamin C infusion may be a significantly effective therapeutic agent in COVID-19 treatment. However there is no clear or convincing scientific evidence that it works for COVID-19 infections and it is not a standard part of treatment for this infection. Casirivimab and imdevimab to be administered together. Early effective treatment of any disease can help avert progression to more serious illness especially for patients at high risk of disease progression and severe illness with the additional benefit of reducing the burden on healthcare systems.
 Source: mercyone.org
Source: mercyone.org
Select Medical City Healthcare locations are offering monoclonal antibody infusions on a very limited basis to patients who have tested positive for COVID-19 and have other high-risk factors. Early effective treatment of any disease can help avert progression to more serious illness especially for patients at high risk of disease progression and severe illness with the additional benefit of reducing the burden on healthcare systems. As with the other monoclonal antibody infusion treatments casirivimab and. They are used to treat diseases. It contains man-made antibodies similar to the antibodies of people who have recovered from COVID.
 Source: catalyst.nejm.org
Source: catalyst.nejm.org
It works by stopping SARS-CoV-2 from spreading in the body. The medicine used during this treatment is called the Regeneron cocktail a mix of two. Patients must be referred by a Medical City Healthcare physician. Sotrovimab EUA issued May 26 2021 Tocilizumab EUA issued June 24 2021 The FDA authorized the use of these monoclonal antibody therapies to treat mild-to-moderate COVID-19 in adults and pediatric patients when both of these apply. What is Monoclonal Antibody Infusion Therapy.
 Source: nature.com
Source: nature.com
Early effective treatment of any disease can help avert progression to more serious illness especially for patients at high risk of disease progression and severe illness with the additional benefit of reducing the burden on healthcare systems. However there is no clear or convincing scientific evidence that it works for COVID-19 infections and it is not a standard part of treatment for this infection. Hospitalized due to COVID-19. COVID INFUSION TREATMENT FAQ What is BamlanivimabEtesevimab Infusion Treatment. It contains man-made antibodies similar to the antibodies of people who have recovered from COVID.
 Source: clinicaltrialsarena.com
Source: clinicaltrialsarena.com
As with the other monoclonal antibody infusion treatments casirivimab and. Antibody infusion treatment for COVID-19 a success in Hancock County A new clinic up and running for eight weeks now is giving patients monoclonal antibody infusion treatments to fight the virus. Health care providers and scientists are investigating other drugs and treatments that may slow or reduce the virus. Clinical trials show that Regenerons monoclonal antibody treatment a combination of two antibodies called casirivimab and imdevimab reduces COVID-19-related hospitalization or deaths in high. COVID INFUSION TREATMENT FAQ What is BamlanivimabEtesevimab Infusion Treatment.
 Source: henryford.com
Source: henryford.com
Antibodies designed to attack COVID-19 have been developed and in several studies have been shown to reduce the risk of progressing to severe COVID-19 and hospitalization when given early to people who test positive for COVID-19. They are used to treat diseases. In response to the COVID-19 PHE the government initially purchased the monoclonal antibody products to treat COVID-19 and made them available for free. Health care providers and scientists are investigating other drugs and treatments that may slow or reduce the virus. Medicare doesnt pay for the monoclonal antibody products to treat COVID-19 that providers get for free including.
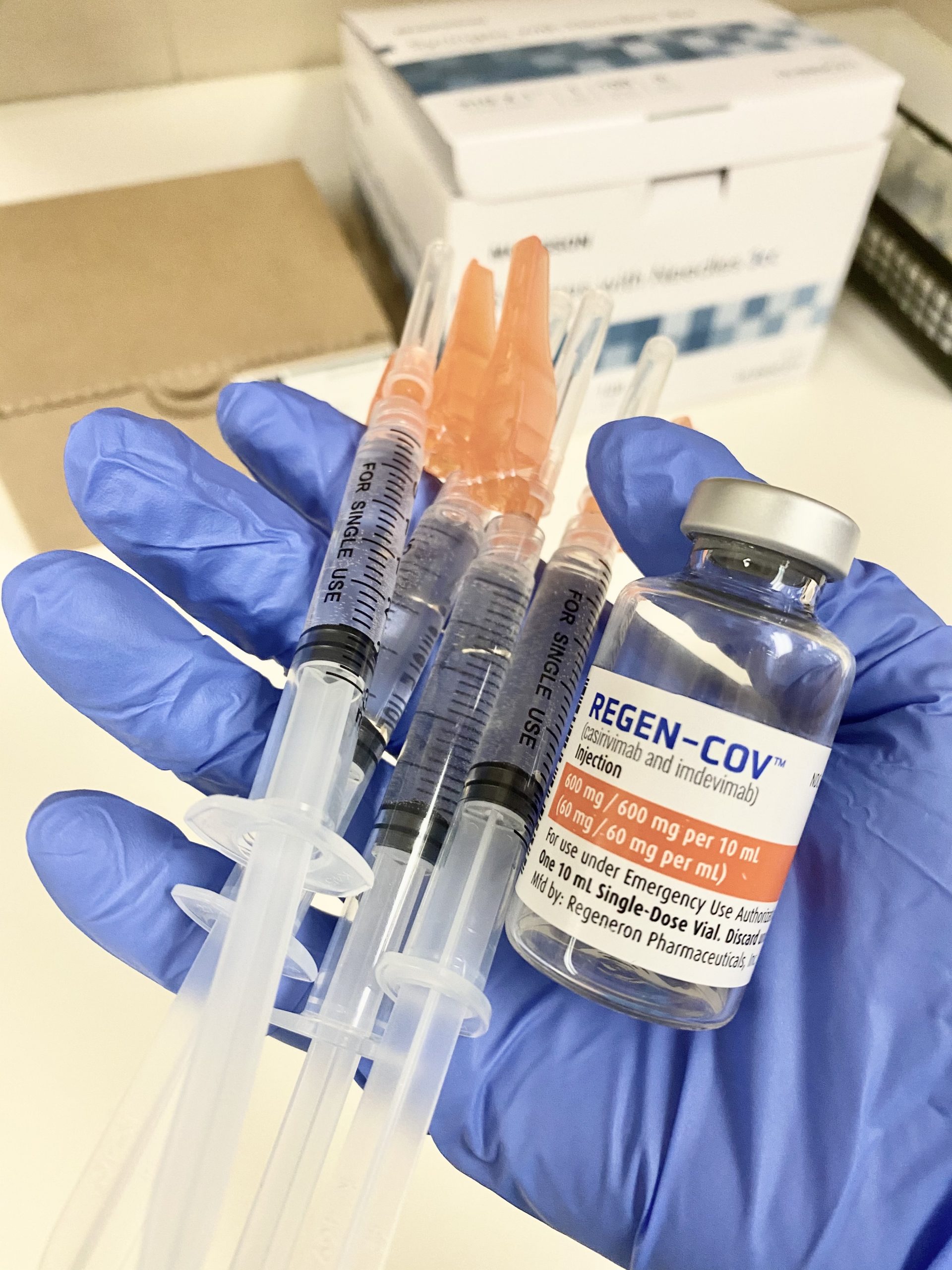 Source: nghs.com
Source: nghs.com
Antibody infusion treatment for COVID-19 a success in Hancock County A new clinic up and running for eight weeks now is giving patients monoclonal antibody infusion treatments to fight the virus. Receiving chronic oxygen therapy due to an underlying non-COVID-19 related comorbidity and require an increase in baseline oxygen flow rate due to COVID-19. Sotrovimab EUA issued May 26 2021 Tocilizumab EUA issued June 24 2021 The FDA authorized the use of these monoclonal antibody therapies to treat mild-to-moderate COVID-19 in adults and pediatric patients when both of these apply. Monoclonal antibody mAb therapy also called monoclonal antibody infusion treatment is a way of treating COVID-19. In response to the COVID-19 PHE the government initially purchased the monoclonal antibody products to treat COVID-19 and made them available for free.
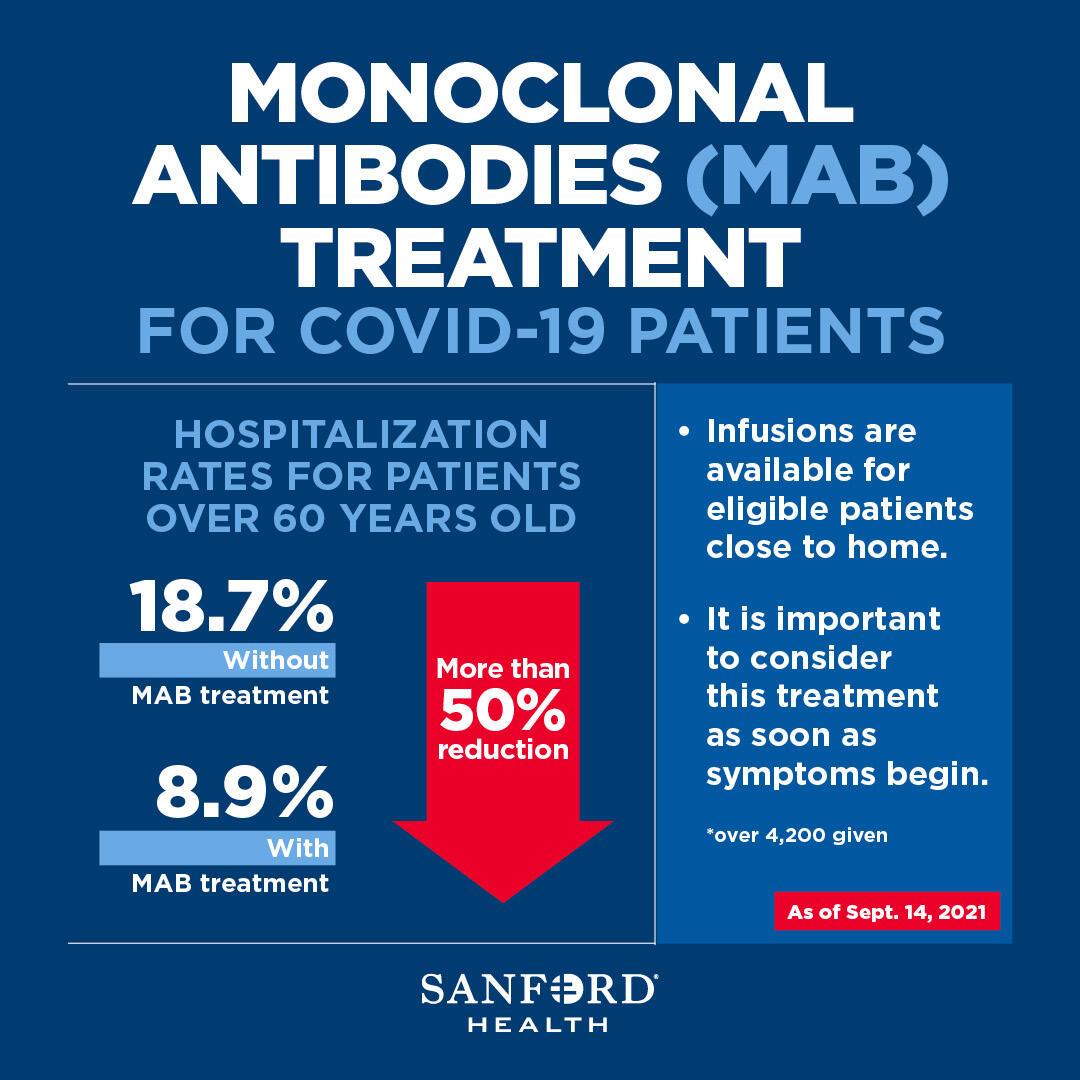 Source: news.sanfordhealth.org
Source: news.sanfordhealth.org
Monoclonal antibody infusion therapy is used to treat a person who has COVID-19. This therapy is given as an infusion through an IV at one of the UNC Health infusion centers. What is Monoclonal Antibody Infusion Therapy. FDA has approved one drug remdesivir Veklury for the treatment of COVID-19 in hospitalized patients aged 12 years and older who weigh at least 40 kg. Early effective treatment of any disease can help avert progression to more serious illness especially for patients at high risk of disease progression and severe illness with the additional benefit of reducing the burden on healthcare systems.
 Source: cidrap.umn.edu
Source: cidrap.umn.edu
The goal of this therapy is to help prevent hospitalizations reduce viral loads and lessen symptom severity. As with the other monoclonal antibody infusion treatments casirivimab and. However there is no clear or convincing scientific evidence that it works for COVID-19 infections and it is not a standard part of treatment for this infection. Receiving oxygen therapy due to COVID-19. Patients must be referred by a Medical City Healthcare physician.

Since there is currently a scarcity of related research whether high-dose vitamin C can improve the prognosis of patients with COVID-19 is yet to be ascertained and is a high-priority concern for medical scholars. Monoclonal Antibody Products to Treat COVID-19 1. It does not contain any COVID-19 virus or parts and does not come from another person who has had COVID. The goal of this therapy is to help prevent hospitalizations reduce viral loads and lessen symptom severity. As with the other monoclonal antibody infusion treatments casirivimab and.
 Source: dukehealth.org
Source: dukehealth.org
Antibody infusion treatment for COVID-19 a success in Hancock County A new clinic up and running for eight weeks now is giving patients monoclonal antibody infusion treatments to fight the virus. In response to the COVID-19 PHE the government initially purchased the monoclonal antibody products to treat COVID-19 and made them available for free. Patients must be referred by a Medical City Healthcare physician. Like your bodys own antibodies monoclonal antibodies recognize specific targets. This therapy is given as an infusion through an IV at one of the UNC Health infusion centers.
 Source: allergyasthmanetwork.org
Source: allergyasthmanetwork.org
Since there is currently a scarcity of related research whether high-dose vitamin C can improve the prognosis of patients with COVID-19 is yet to be ascertained and is a high-priority concern for medical scholars. COVID INFUSION TREATMENT FAQ What is BamlanivimabEtesevimab Infusion Treatment. Select Medical City Healthcare locations are offering monoclonal antibody infusions on a very limited basis to patients who have tested positive for COVID-19 and have other high-risk factors. Remdesivir is an antiviral drug approved by the FDA for the treatment of COVID-19 in hospitalized adults and hospitalized pediatric patients at least 12 years of age. It works by stopping SARS-CoV-2 from spreading in the body.
 Source: nytimes.com
Source: nytimes.com
The treatment takes about an hour for the infusion and a little under that time for patient observation once the treatment is completed. Patients must be referred by a Medical City Healthcare physician. The patient has a positive COVID-19 test result. Monoclonal Antibody Products to Treat COVID-19 1. Since there is currently a scarcity of related research whether high-dose vitamin C can improve the prognosis of patients with COVID-19 is yet to be ascertained and is a high-priority concern for medical scholars.
 Source: health.ucdavis.edu
Source: health.ucdavis.edu
COVID-19 Monoclonal Antibody Therapy Infusion For Patients If you have recently been diagnosed with COVID-19 there may be treatment options available to you. Monoclonal Antibody COVID-19 Infusion. It does not contain any COVID-19 virus or parts and does not come from another person who has had COVID. Monoclonal antibody mAb therapy also called monoclonal antibody infusion treatment is a way of treating COVID-19. What is Monoclonal Antibody Infusion Therapy.
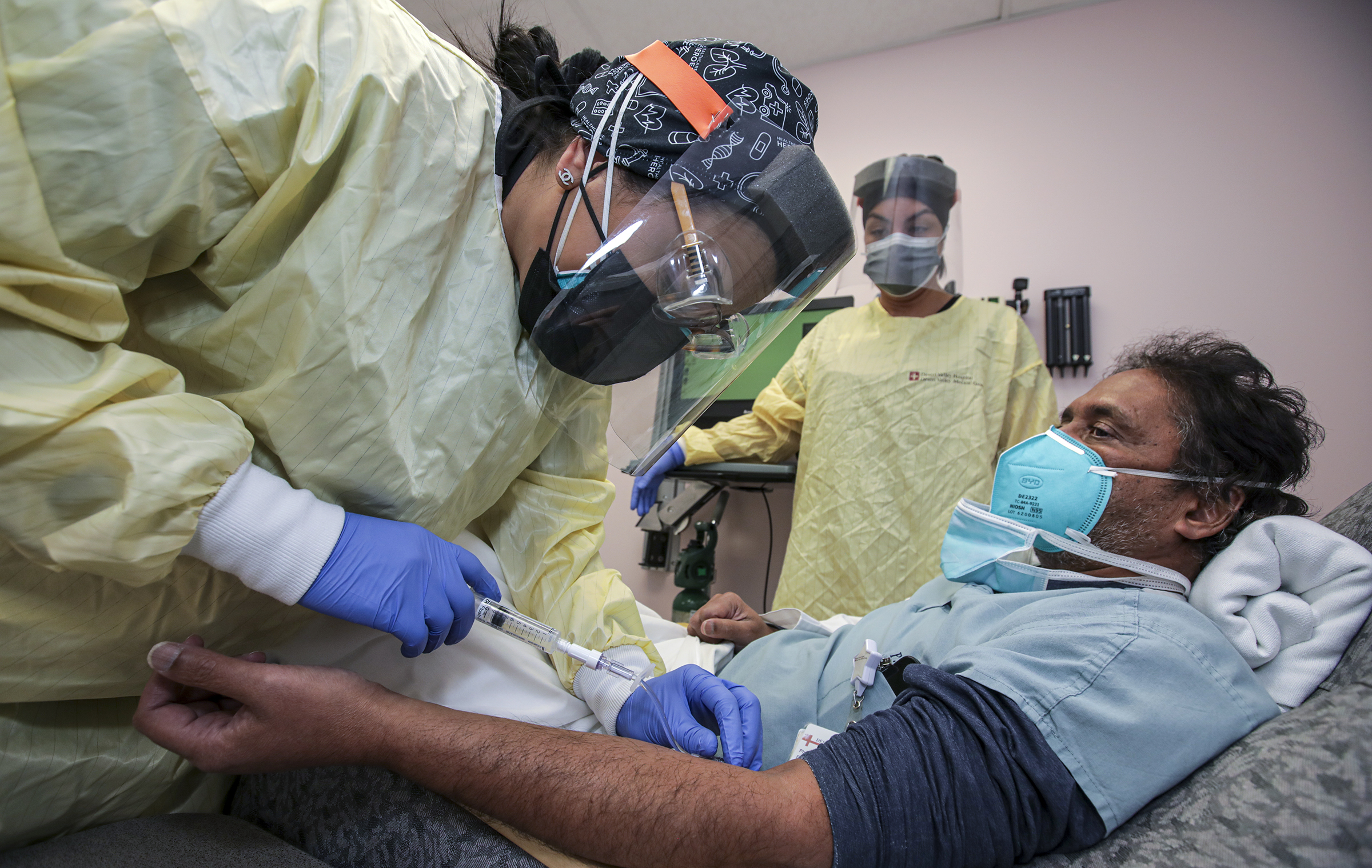 Source: npr.org
Source: npr.org
The treatment takes about an hour for the infusion and a little under that time for patient observation once the treatment is completed. Sotrovimab EUA issued May 26 2021 Tocilizumab EUA issued June 24 2021 The FDA authorized the use of these monoclonal antibody therapies to treat mild-to-moderate COVID-19 in adults and pediatric patients when both of these apply. It works by stopping SARS-CoV-2 from spreading in the body. FDA has approved one drug remdesivir Veklury for the treatment of COVID-19 in hospitalized patients aged 12 years and older who weigh at least 40 kg. As with the other monoclonal antibody infusion treatments casirivimab and.
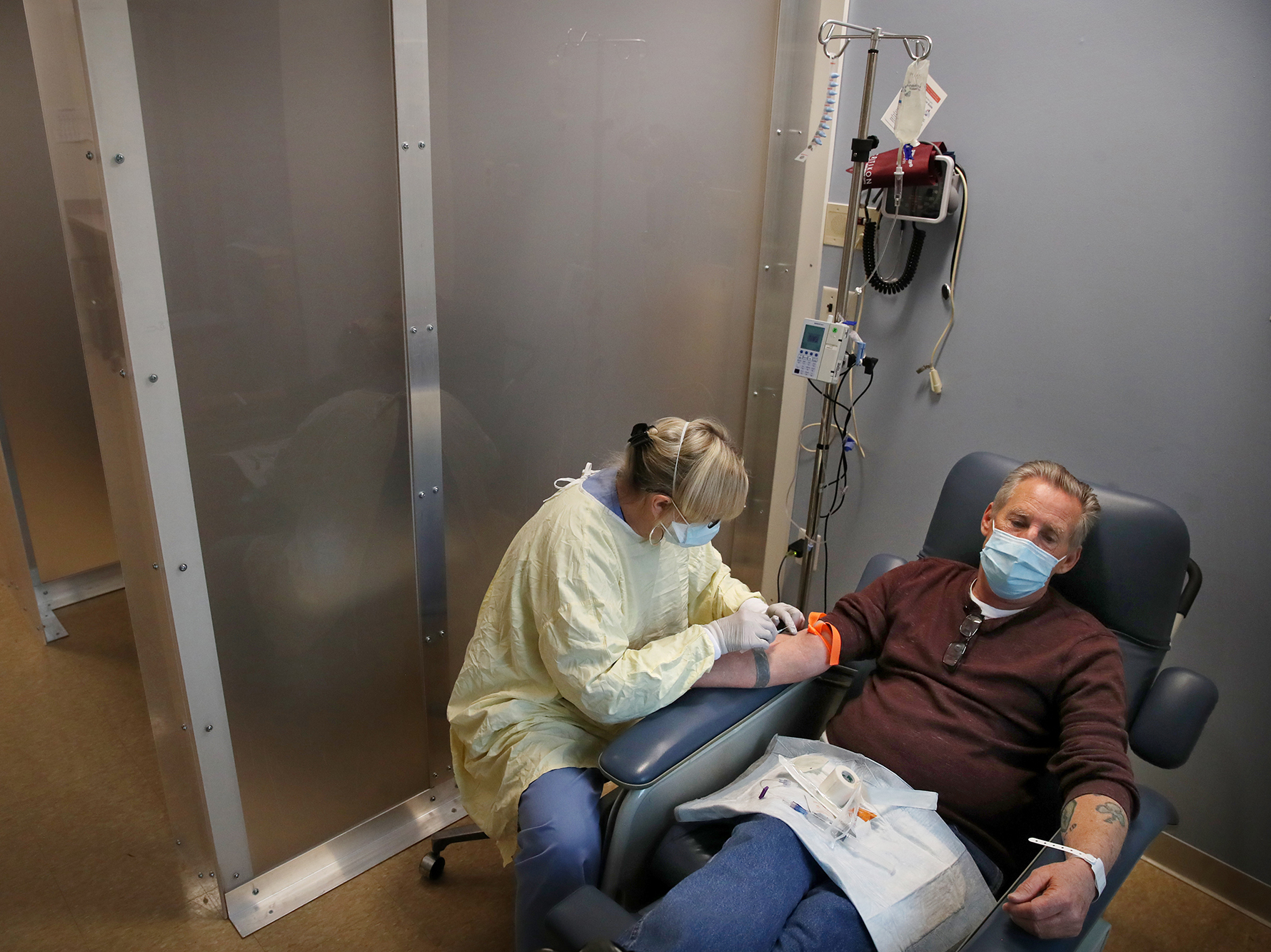 Source: npr.org
Source: npr.org
Casirivimab and imdevimab to be administered together. MedStar Health is offering a treatment known as monoclonal antibody infusion to help patients who have been diagnosed with COVID-19 and are at risk of developing a severe case of the illness. It works by stopping SARS-CoV-2 from spreading in the body. Since there is currently a scarcity of related research whether high-dose vitamin C can improve the prognosis of patients with COVID-19 is yet to be ascertained and is a high-priority concern for medical scholars. Antibodies designed to attack COVID-19 have been developed and in several studies have been shown to reduce the risk of progressing to severe COVID-19 and hospitalization when given early to people who test positive for COVID-19.
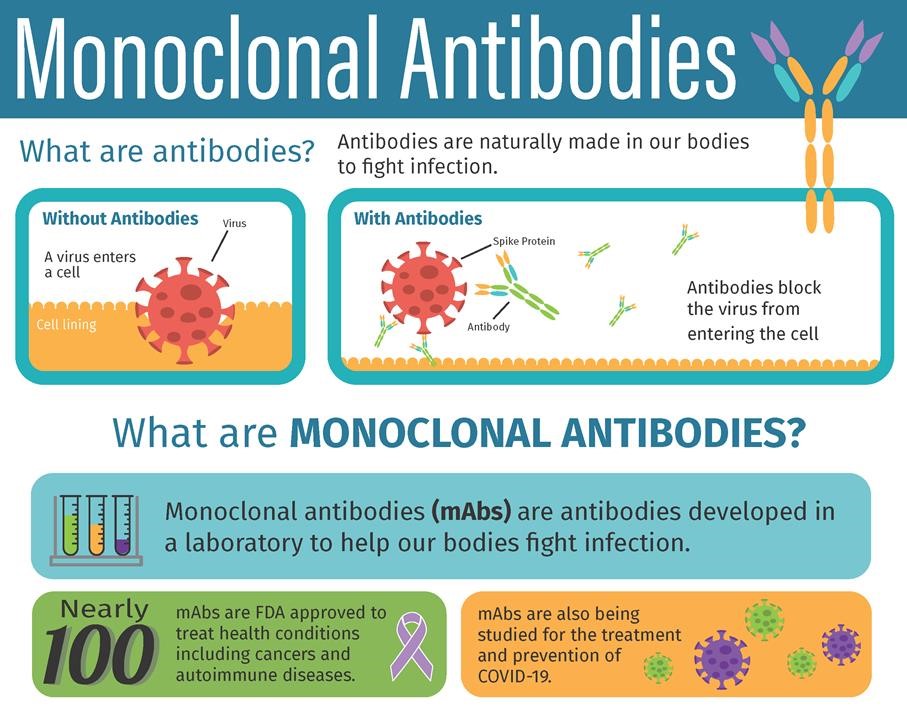 Source: deaconess.com
Source: deaconess.com
Antibodies designed to attack COVID-19 have been developed and in several studies have been shown to reduce the risk of progressing to severe COVID-19 and hospitalization when given early to people who test positive for COVID-19. Antibodies designed to attack COVID-19 have been developed and in several studies have been shown to reduce the risk of progressing to severe COVID-19 and hospitalization when given early to people who test positive for COVID-19. Remdesivir is an antiviral drug approved by the FDA for the treatment of COVID-19 in hospitalized adults and hospitalized pediatric patients at least 12 years of age. Therapy casirivimab and imdevimab administered t ogether for the treatment of mild-to -moderate COVID -19 in adults and pediatric patients with postivie COVID -19 test results who are at high risk for progressngi to severe COVID-19 andor hospitalization. In addition to outpatient treatments on June 24 2021 the FDA granted an EUA for a recombinant humanized monoclonal antibody tTocilizumab for certain hospitalized COVID-19 patients.
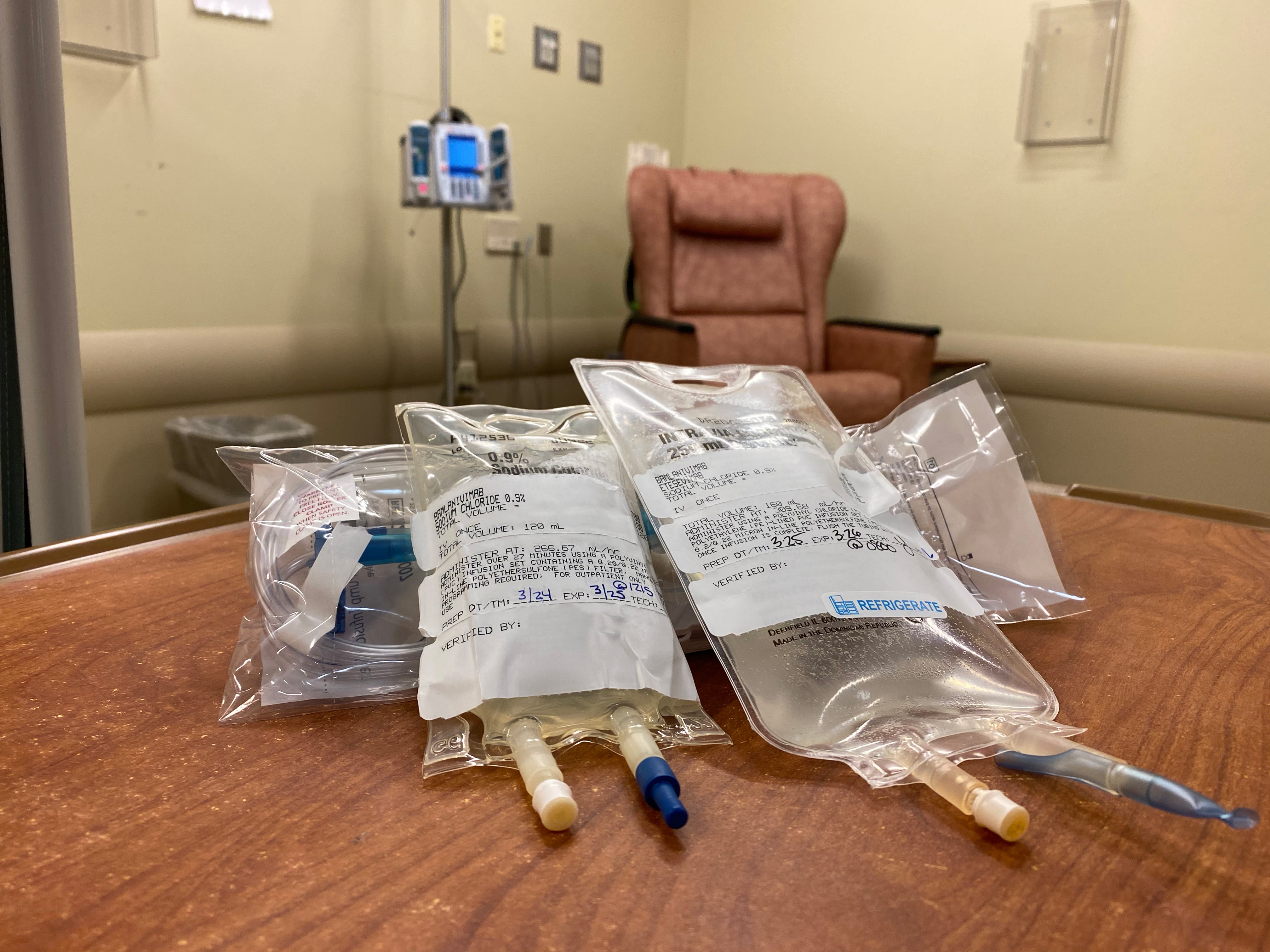 Source: cleveland.com
Source: cleveland.com
Monoclonal antibody mAb therapy also called monoclonal antibody infusion treatment is a way of treating COVID-19. Antibody infusion treatment for COVID-19 a success in Hancock County A new clinic up and running for eight weeks now is giving patients monoclonal antibody infusion treatments to fight the virus. Like your bodys own antibodies monoclonal antibodies recognize specific targets. Remdesivir is an antiviral drug approved by the FDA for the treatment of COVID-19 in hospitalized adults and hospitalized pediatric patients at least 12 years of age. Medicare doesnt pay for the monoclonal antibody products to treat COVID-19 that providers get for free including.
This site is an open community for users to submit their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site adventageous, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title treatment for covid 19 infusion by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.





