Your Remdesivir for treatment of ebola images are available. Remdesivir for treatment of ebola are a topic that is being searched for and liked by netizens today. You can Get the Remdesivir for treatment of ebola files here. Find and Download all free images.
If you’re looking for remdesivir for treatment of ebola pictures information linked to the remdesivir for treatment of ebola interest, you have visit the right blog. Our website always provides you with suggestions for viewing the maximum quality video and picture content, please kindly surf and locate more informative video articles and images that fit your interests.
Remdesivir For Treatment Of Ebola. Remdesivir went from potential Ebola treatment to offering modest benefits for people with COVID-19. Initial animal experiments seemed to show the drug could work against Ebola leading Gilead to start Phase 1 testing in 2015 and advance it into a mid-stage trial the next year. The researchers randomly assigned participants to receive one of four experimental drugs. Although remdesivir performed well in preclinical studies it did not meet efficacy endpoints in a randomized trial conducted during an Ebola outbreak.
 The Strange Story Of Remdesivir A Covid Drug That Doesn T Work From forbes.com
The Strange Story Of Remdesivir A Covid Drug That Doesn T Work From forbes.com
Remdesivir emerged from work done by Gilead and US. Fast forward to the Chinese study. Drugmaker Gilead Sciences Inc. Remdesivir an antiviral drug developed during the Ebola crisis in Western Africa in 2015 has been evaluated as a possible treatment for those infected with the coronavirus in multiple clinical. 35 61174 of patients in the mAb114 treatment group and 34 52155 of patients in the REGN-EB3 group died by 28 days post-treatment. Began research on remdesivir in 2009.
The mortality rate in the remdesivir treatment group 53 93175 was similar to ZMapp.
Remdesivir emerged from work done by Gilead and US. Remdesivir was originally created and developed by Gilead Sciences in 2009 to treat hepatitis C and respiratory syncytial virus RSV. Randomized Controlled Trial of Ebola Treatments Ebola transmission has been ongoing in the Democratic Republic of Congo since August 2018. Remdesivir holds promise for treating COVID-19 based on in vitro activity. One leading candidate is remdesivir GS-5734 a broad-spectrum antiviral that was initially developed for the treatment of Ebola virus EBOV. Remdesivir which is administered intravenously was among the first drugs suggested as a treatment for the novel coronavirus and as such has great hopes riding on it.
 Source: nytimes.com
Source: nytimes.com
Drugmaker Gilead Sciences Inc. Began research on remdesivir in 2009. Though remdesivir failed to help people with Ebola virus disease encouraging results from studies of coronavirus-infected animals have prompted the launch of human clinical trials to see if this drug might fight SARS-CoV-2 the novel coronavirus. Participants could be any age and included infants and pregnant women. Later testing showed that.
 Source: frontiersin.org
Source: frontiersin.org
The treatments the antiviral remdesivir and antibodies in the. The Congo Ebola clinical trial was sponsored by the National Institute of Allergy and Infectious Diseases of which Fauci is the director. Remdesivir an antiviral drug developed during the Ebola crisis in Western Africa in 2015 has been evaluated as a possible treatment for those infected with the coronavirus in multiple clinical. Mortality rates were lower for mAb114 and REGN-EB3 compared to their respective ZMapp cohorts. One patient died after receiving remdesivir.
 Source: clinicaltrialsarena.com
Source: clinicaltrialsarena.com
Stephen Evans a professor of pharmacoepidemiology at the London School of Hygiene Tropical Medicine who was not involved in the research said the trial was too small in numbers. One patient died after receiving remdesivir. Remdesivir which is administered intravenously was among the first drugs suggested as a treatment for the novel coronavirus and as such has great hopes riding on it. So lets focus on an experimental anti-viral drug called remdesivir that was originally developed for the deadly Ebola virus. Participants could be any age and included infants and pregnant women.
 Source: nejm.org
Source: nejm.org
Remdesivir which is administered intravenously was among the first drugs suggested as a treatment for the novel coronavirus and as such has great hopes riding on it. It did not work against hepatitis C or RSV but was then repurposed and studied as a potential treatment for Ebola. One patient died after receiving remdesivir. ZMapp Mab114 REGN-EB3 or remdesivir. Mortality rates were lower for mAb114 and REGN-EB3 compared to their respective ZMapp cohorts.
 Source: clinicaltrialsarena.com
Source: clinicaltrialsarena.com
Ardis says that Remdesivir was by far the least safe and effective of the four drugs in the Ebola trial and that the one-year study was cancelled by safety monitors in August of 2019 after just 6 months because of a 531 mortality rate not from ineffectively treated Ebola but from kidney and other organ failure caused by the drug itself. So lets focus on an experimental anti-viral drug called remdesivir that was originally developed for the deadly Ebola virus. In remdesivirs case thats the virus genetic material RNA. Ardis says that Remdesivir was by far the least safe and effective of the four drugs in the Ebola trial and that the one-year study was cancelled by safety monitors in August of 2019 after just 6 months because of a 531 mortality rate not from ineffectively treated Ebola but from kidney and other organ failure caused by the drug itself. Initial animal experiments seemed to show the drug could work against Ebola leading Gilead to start Phase 1 testing in 2015 and advance it into a mid-stage trial the next year.
 Source: nbcnews.com
Source: nbcnews.com
NIAID is studying its ability to clear Ebola virus RNA from the semen of Ebola survivors in a study in Liberia known as PREVAIL 4. The mortality rate in the remdesivir treatment group was 53 the highest death rate of the drugs being tested. Gilead scientists are working to help change that with an investigational agent called remdesivir GS. 35 61174 of patients in the mAb114 treatment group and 34 52155 of patients in the REGN-EB3 group died by 28 days post-treatment. Later testing showed that.
 Source: newscientist.com
Source: newscientist.com
One patient died after receiving remdesivir. Though remdesivir failed to help people with Ebola virus disease encouraging results from studies of coronavirus-infected animals have prompted the launch of human clinical trials to see if this drug might fight SARS-CoV-2 the novel coronavirus. Gilead scientists are working to help change that with an investigational agent called remdesivir GS. Mortality rates were lower for mAb114 and REGN-EB3 compared to their respective ZMapp cohorts. The mortality rate in the remdesivir treatment group 53 93175 was similar to ZMapp.
 Source: abcnews.go.com
Source: abcnews.go.com
Remdesivir was originally created and developed by Gilead Sciences in 2009 to treat hepatitis C and respiratory syncytial virus RSV. Drugmaker Gilead Sciences Inc. The collaboration identified as Remdesivir GS-5734 is a nucleotide prodrug that is processed in the body to rapidly release the active drug into cells. In remdesivirs case thats the virus genetic material RNA. One patient died after receiving remdesivir.
 Source: pharmaceutical-technology.com
Source: pharmaceutical-technology.com
The Congo Ebola clinical trial was sponsored by the National Institute of Allergy and Infectious Diseases of which Fauci is the director. Fast forward to the Chinese study. Remdesivir was originally created and developed by Gilead Sciences in 2009 to treat hepatitis C and respiratory syncytial virus RSV. This story was updated at 1237 pm EDT to include information on remdesivir treatment in monkeys. 35 61174 of patients in the mAb114 treatment group and 34 52155 of patients in the REGN-EB3 group died by 28 days post-treatment.

The Congo Ebola clinical trial was sponsored by the National Institute of Allergy and Infectious Diseases of which Fauci is the director. One patient died after receiving remdesivir. The mortality rate in the remdesivir treatment group 53 93175 was similar to ZMapp. It did not work against hepatitis C or RSV but was then repurposed and studied as a potential treatment for Ebola. Initial animal experiments seemed to show the drug could work against Ebola leading Gilead to start Phase 1 testing in 2015 and advance it into a mid-stage trial the next year.
 Source: indianexpress.com
Source: indianexpress.com
Participants could be any age and included infants and pregnant women. The Congo Ebola clinical trial was sponsored by the National Institute of Allergy and Infectious Diseases of which Fauci is the director. The investigational antiviral agent GS-5734 also known as remdesivir is being developed by Gilead as a treatment for Ebola virus disease. Remdesivir an antiviral drug developed during the Ebola crisis in Western Africa in 2015 has been evaluated as a possible treatment for those infected with the coronavirus in multiple clinical. Began research on remdesivir in 2009.
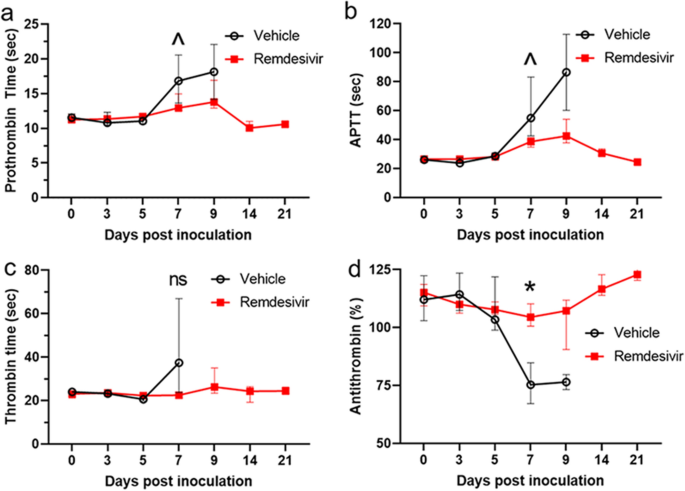 Source: nature.com
Source: nature.com
The mortality rate in the remdesivir treatment group 53 93175 was similar to ZMapp. Specifically the FDA agreed that the rhesus macaque infected by intramuscular IM injection is a relevant and adequately characterized model of Ebola virus disease to support filing under. Participants could be any age and included infants and pregnant women. New research by the Centers for Disease Control and Prevention CDC shows two investigational Ebola treatments being used in the ongoing outbreak in eastern Democratic Republic of the Congo DRC are effective in laboratory studies. Ardis says that Remdesivir was by far the least safe and effective of the four drugs in the Ebola trial and that the one-year study was cancelled by safety monitors in August of 2019 after just 6 months because of a 531 mortality rate not from ineffectively treated Ebola but from kidney and other organ failure caused by the drug itself.
 Source: pharmaceutical-journal.com
Source: pharmaceutical-journal.com
Remdesivir went from potential Ebola treatment to offering modest benefits for people with COVID-19. The mortality rate in the remdesivir treatment group 53 93175 was similar to ZMapp. The collaboration identified as Remdesivir GS-5734 is a nucleotide prodrug that is processed in the body to rapidly release the active drug into cells. Participants could be any age and included infants and pregnant women. Began research on remdesivir in 2009.
 Source: cardiff.ac.uk
Source: cardiff.ac.uk
The collaboration identified as Remdesivir GS-5734 is a nucleotide prodrug that is processed in the body to rapidly release the active drug into cells. Although remdesivir performed well in preclinical studies it did not meet efficacy endpoints in a randomized trial conducted during an Ebola outbreak. A relatively new emerging virus Ebola currently has no approved treatment and depending on virus strain and kind of healthcare available it kills 40 to 80 percent of those infected. The mortality rate in the remdesivir treatment group 53 93175 was similar to ZMapp. Began research on remdesivir in 2009.
 Source: ft.com
Source: ft.com
However remdesivir a nucleotide analogue prodrug originally developed for the treatment of Ebola virus was found to inhibit the replication of a wide range of human and animal coronaviruses in vitro and in preclinical studies. The mortality rate in the remdesivir treatment group was 53 the highest death rate of the drugs being tested. Initial animal experiments seemed to show the drug could work against Ebola leading Gilead to start Phase 1 testing in 2015 and advance it into a mid-stage trial the next year. A relatively new emerging virus Ebola currently has no approved treatment and depending on virus strain and kind of healthcare available it kills 40 to 80 percent of those infected. One leading candidate is remdesivir GS-5734 a broad-spectrum antiviral that was initially developed for the treatment of Ebola virus EBOV.
 Source: statnews.com
Source: statnews.com
Randomized Controlled Trial of Ebola Treatments Ebola transmission has been ongoing in the Democratic Republic of Congo since August 2018. A relatively new emerging virus Ebola currently has no approved treatment and depending on virus strain and kind of healthcare available it kills 40 to 80 percent of those infected. One leading candidate is remdesivir GS-5734 a broad-spectrum antiviral that was initially developed for the treatment of Ebola virus EBOV. Ardis says that Remdesivir was by far the least safe and effective of the four drugs in the Ebola trial and that the one-year study was cancelled by safety monitors in August of 2019 after just 6 months because of a 531 mortality rate not from ineffectively treated Ebola but from kidney and other organ failure caused by the drug itself. Randomized Controlled Trial of Ebola Treatments Ebola transmission has been ongoing in the Democratic Republic of Congo since August 2018.
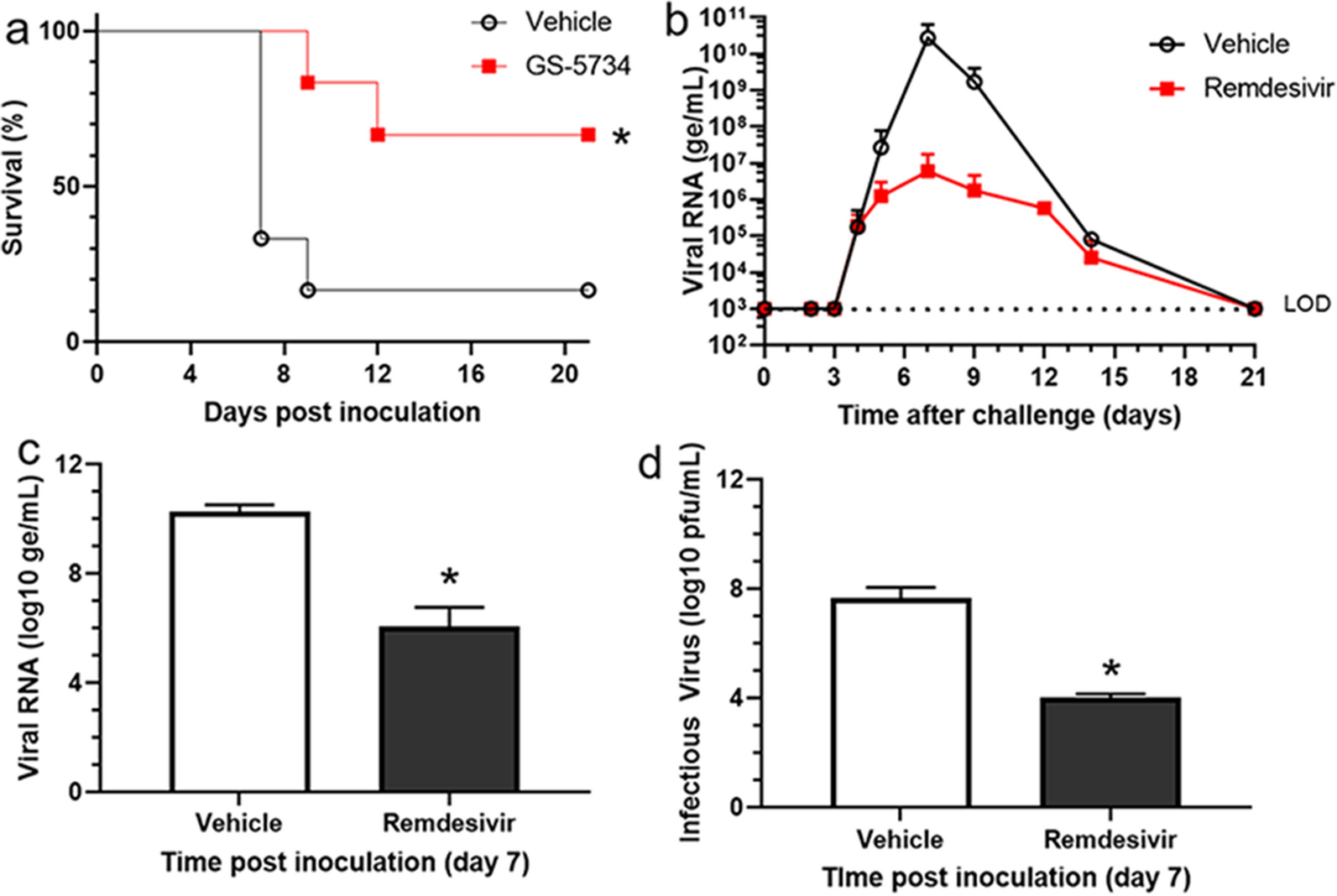 Source: nature.com
Source: nature.com
One leading candidate is remdesivir GS-5734 a broad-spectrum antiviral that was initially developed for the treatment of Ebola virus EBOV. One leading candidate is remdesivir GS-5734 a broad-spectrum antiviral that was initially developed for the treatment of Ebola virus EBOV. Stephen Evans a professor of pharmacoepidemiology at the London School of Hygiene Tropical Medicine who was not involved in the research said the trial was too small in numbers. This story was updated at 1237 pm EDT to include information on remdesivir treatment in monkeys. The drug proved ineffective against the Ebola virus however yet was still subsequently repurposed for SARS-CoV-2.
 Source: sciencedirect.com
Source: sciencedirect.com
Remdesivir holds promise for treating COVID-19 based on in vitro activity. One patient died after receiving remdesivir. This story was updated at 1237 pm EDT to include information on remdesivir treatment in monkeys. New research by the Centers for Disease Control and Prevention CDC shows two investigational Ebola treatments being used in the ongoing outbreak in eastern Democratic Republic of the Congo DRC are effective in laboratory studies. Later testing showed that.
This site is an open community for users to do submittion their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site serviceableness, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title remdesivir for treatment of ebola by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.





