Your Nih covid treatment table 2e images are ready. Nih covid treatment table 2e are a topic that is being searched for and liked by netizens today. You can Download the Nih covid treatment table 2e files here. Get all royalty-free photos.
If you’re looking for nih covid treatment table 2e pictures information related to the nih covid treatment table 2e keyword, you have come to the right site. Our website always gives you suggestions for viewing the highest quality video and image content, please kindly hunt and find more informative video content and graphics that match your interests.
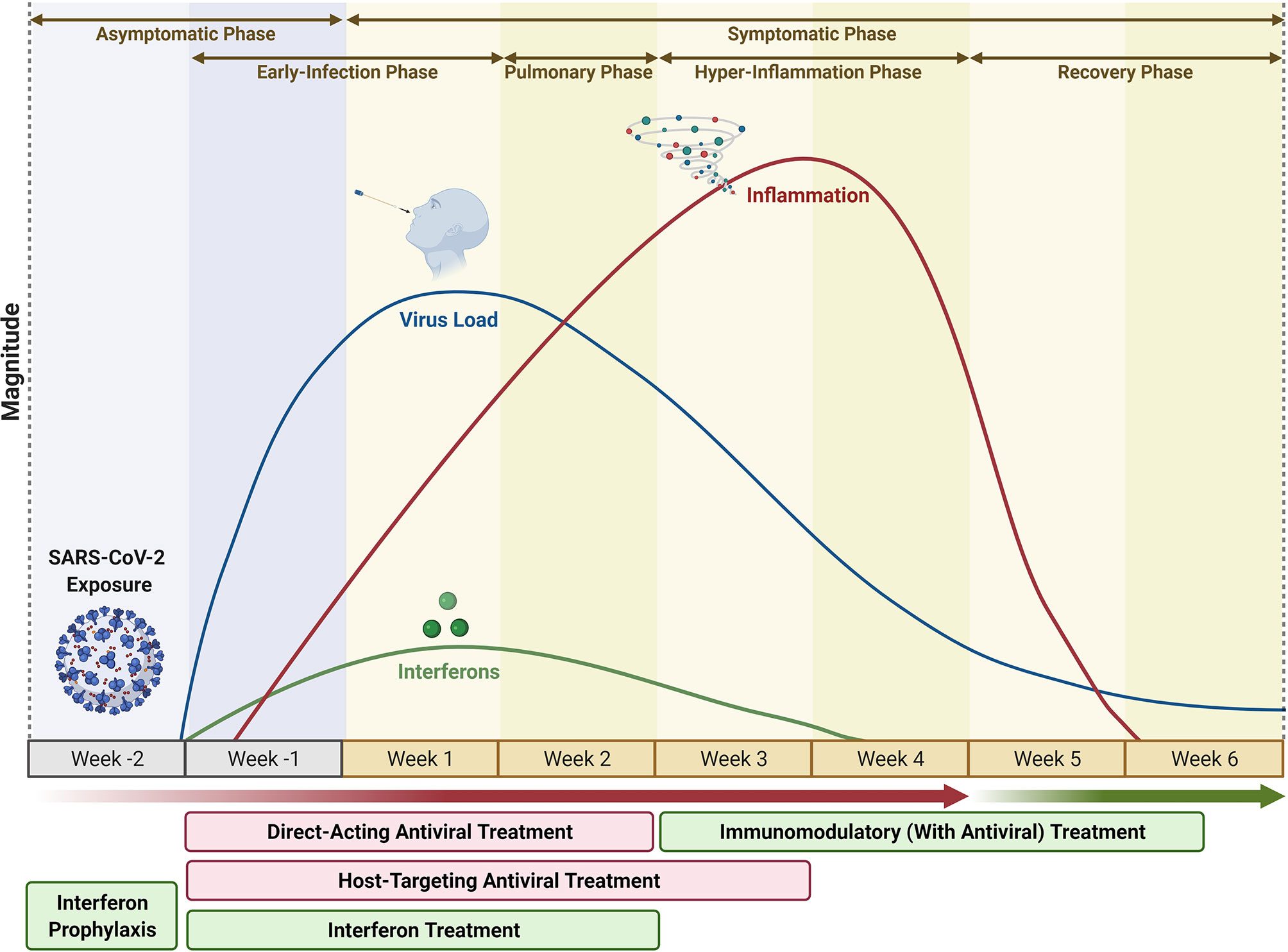
Nih Covid Treatment Table 2e. NIH is leading and supporting research on safe and effective treatments to fight COVID-19. All patients 71 male had a confirmed SARS-CoV-2 infection and signs symptoms and radiological findings compatible with COVID-19 pneumonia and all of them underwent a trial of NIV. Please visit the NIH ACTIV vaccines page for a summary of Operation Warp Speed-supported clinical trials of COVID-19 vaccine candidates. COVID-19 Treatment Guidelines Panel the Panel provides recommendations for using antiviral drugs to treat COVID-19 based on the available data.
 Drug Repurposing Clinical Trials In The Search For Life Saving Covid 19 Therapies Research Targets And Methodological And Ethical Issues From redalyc.org
Drug Repurposing Clinical Trials In The Search For Life Saving Covid 19 Therapies Research Targets And Methodological And Ethical Issues From redalyc.org
The candidate vaccines developed by AstraZeneca and Oxford AZD1222 and the Janssen Pharmaceutical Companies of Johnson Johnson JNJ-7843672 or Ad26COV2S are viral vector-based. The table below lists and links to data collection instruments including surveys for assessing COVID-19-relevant Behavioral and Social Science BSSR domains for clinical or population research. Persons fully vaccinated per 100 population. Ivermectin is APPROVED by the NIH as a treatment for COVID-19. In the study more than 6000 patients hospitalized with COVID-19 randomly received either dexamethasone or standard treatment. For more information on these antiviral agents see Table 2e.
Some of these purported remedies include teas essential oils vitamins tinctures herbal therapies such as oleanderoleandrin and silver products such as colloidal silver.
COVID-19 Treatment Guidelines Panel the Panel provides recommendations for using antiviral drugs to treat COVID-19 based on the available data. Characteristics of Antiviral Agents That Are Approved or Under. Tracey Rouault from NIHs Eunice Kennedy Shriver National Institute of Child Health and Human Development NICHD looked for new ways to target an enzyme produced by SARS-CoV-2. The table below lists and links to data collection instruments including surveys for assessing COVID-19-relevant Behavioral and Social Science BSSR domains for clinical or population research. Our COVID-19 research response is helping to create safe and accurate tests treatments and vaccines. Disease severity Potential Treatment Recommendations per ID consult discretion based on details in Table 2 Post-exposure prophylaxis See -Exposure Prophylaxis Guidelines No supplemental oxygen Supportive care Monoclonal Antibodies may be an option in certain high-risk patients see eligibility criteria in Table 2 admitted for reasons other than COVID-19 who.
 Source: frontiersin.org
Source: frontiersin.org
While a vaccine is a central part of the effort to prevent infection and illness there is no specific antiviral treatment recommended for COVID-19. COVID-19 can also cause other complications. 157 Anti-SARS-CoV-2 Monoclonal Antibodies. For severe cases treatment should include oxygen supplementation and care to support vital organ functions as needed. Characteristics of Antiviral Agents That Are Approved or Under.

While a vaccine is a central part of the effort to prevent infection and illness there is no specific antiviral treatment recommended for COVID-19. Tracey Rouault from NIHs Eunice Kennedy Shriver National Institute of Child Health and Human Development NICHD looked for new ways to target an enzyme produced by SARS-CoV-2. The NIH COVID-19 treatment guidelines recommend the use of dexamethasone in certain people hospitalized with severe COVID-19. Do you know the difference between FDA approval and authorization. Our COVID-19 research response is helping to create safe and accurate tests treatments and vaccines.
 Source: resmedjournal.com
Source: resmedjournal.com
NIH is leading and supporting research on safe and effective treatments to fight COVID-19. The pan-el also recommends against use of high-dose chloroquine ie 600 mg twice daily for 10 days for the treatment of COVID-19 because such dosage has been associated with more severe toxic-ities compared with lower-dose chloro-quine. Ivermectin is an antiparasitic drug being investigated for repurposing against severe acute respiratory syndrome coronavirus 2 SARS-CoV-2. The recommendation was based on results from the RECOVERY trial. 4E2 Thyroid 1E3 7E2 Thyroid 2E3 3E-7.

Table 2e - Characteristics of Antiviral Agents that are Approved or Under Evaluation for the Treatment of COVID-19. To treat outpatients with mild COVID-19. Please visit the NIH ACTIV vaccines page for a summary of Operation Warp Speed-supported clinical trials of COVID-19 vaccine candidates. Mortality clinical worsening or improvement adverse events quality of life duration of hospitalization and viral clearance. Primary outcomes were NIV success and failure defined by intubation and mortality rate.
 Source: frontiersin.org
Source: frontiersin.org
People with COVID-19 should receive support to help relieve symptoms. An approval of a new drug requires substantial scientific evidence of the effectiveness of the product and a demonstration of safety for the drugs intended uses. Together this information lets scientists search for new drugs to treat COVID-19 that are targeted specifically to its structure and functions. The candidate vaccines developed by AstraZeneca and Oxford AZD1222 and the Janssen Pharmaceutical Companies of Johnson Johnson JNJ-7843672 or Ad26COV2S are viral vector-based. Aug 18 2021 R01.
 Source: covid19treatmentguidelines.nih.gov
Source: covid19treatmentguidelines.nih.gov
In a new study a research team led by Dr. Together this information lets scientists search for new drugs to treat COVID-19 that are targeted specifically to its structure and functions. 4E4 Stomach Wall 6E4 1E5. All patients 71 male had a confirmed SARS-CoV-2 infection and signs symptoms and radiological findings compatible with COVID-19 pneumonia and all of them underwent a trial of NIV. Patients who are hospitalized with serious COVID-19 illness might also be given blood thinners to prevent or treat blood clots.

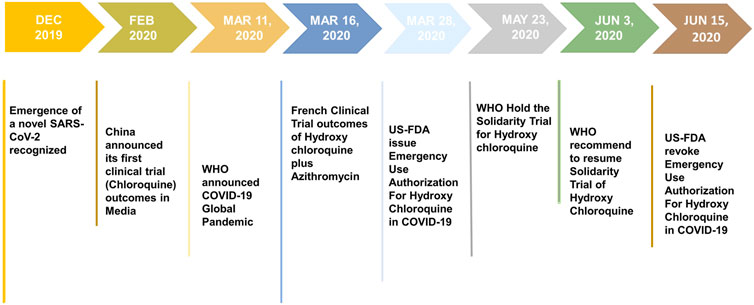
Our COVID-19 research response is helping to create safe and accurate tests treatments and vaccines. Without azithromycin for the treatment of COVID-19 in nonhospitalized pa-tients except in a clinical trial. The databases Medline Embase and WHO COVID-19 were searched for all publications from 2019 to 9 February 2021 using a combination of the terms COVID 19 SARSCoV2 coronavirus therapy lopinavir ritonavir ribavirin remdesivir hydroxychloroquine corticosteroids anakinra tocilizumab convalescent plasma. Some of these purported remedies include teas essential oils vitamins tinctures herbal therapies such as oleanderoleandrin and silver products such as colloidal silver. Mortality clinical worsening or improvement admission to hospital adverse events.
 Source: thelancet.com
Source: thelancet.com
157 Anti-SARS-CoV-2 Monoclonal Antibodies. As in the management of any disease treatment decisions ultimately reside with the patient and their health care provider. All patients 71 male had a confirmed SARS-CoV-2 infection and signs symptoms and radiological findings compatible with COVID-19 pneumonia and all of them underwent a trial of NIV. NIH is leading and supporting research on safe and effective treatments to fight COVID-19. Patients who are hospitalized with serious COVID-19 illness might also be given blood thinners to prevent or treat blood clots.
 Source: mayoclinicproceedings.org
Source: mayoclinicproceedings.org
5E0 Thyroid 2E1 9E0 Thyroid 3E1 4E-9. 157 Anti-SARS-CoV-2 Monoclonal Antibodies. 3E1 Thyroid 9E1 5E1 Thyroid 2E2 2E-8. As in the management of any disease treatment decisions ultimately reside with the patient and their health care provider. Please visit the NIH ACTIV vaccines page for a summary of Operation Warp Speed-supported clinical trials of COVID-19 vaccine candidates.
 Source: ackdjournal.org
Source: ackdjournal.org
In the study more than 6000 patients hospitalized with COVID-19 randomly received either dexamethasone or standard treatment. In a new study a research team led by Dr. Primary outcomes were NIV success and failure defined by intubation and mortality rate. For more information on these antiviral agents see Table 2e. Patients who are hospitalized with serious COVID-19 illness might also be given blood thinners to prevent or treat blood clots.
 Source: frontiersin.org
Source: frontiersin.org
Characteristics of Antiviral Agents That Are Approved or Under. In a new study a research team led by Dr. Ivermectin is an antiparasitic drug being investigated for repurposing against severe acute respiratory syndrome coronavirus 2 SARS-CoV-2. Ivermectin IS for COVID Table 2e. Do you know the difference between FDA approval and authorization.
 Source: redalyc.org
Source: redalyc.org
Ivermectin IS for COVID Table 2e. 153 Anti-SARS-CoV-2 Antibody Products. 2E1 Thyroid 7E1 4E1 Thyroid 1E2 1E-8. Rapid Acceleration of Diagnostics RADx An initiative to speed innovation in the development commercialization and implementation of technologies for COVID-19 testing. To treat inpatients with moderate-to-severe COVID-19.

In the study more than 6000 patients hospitalized with COVID-19 randomly received either dexamethasone or standard treatment. 153 Anti-SARS-CoV-2 Antibody Products. Rapid Acceleration of Diagnostics RADx An initiative to speed innovation in the development commercialization and implementation of technologies for COVID-19 testing. The NIH COVID-19 treatment guidelines recommend the use of dexamethasone in certain people hospitalized with severe COVID-19. Do you know the difference between FDA approval and authorization.

Persons fully vaccinated per 100 population. 157 Anti-SARS-CoV-2 Monoclonal Antibodies. Disease severity Potential Treatment Recommendations per ID consult discretion based on details in Table 2 Post-exposure prophylaxis See -Exposure Prophylaxis Guidelines No supplemental oxygen Supportive care Monoclonal Antibodies may be an option in certain high-risk patients see eligibility criteria in Table 2 admitted for reasons other than COVID-19 who. We used GRADE to rate the certainty of evidence for the following outcomes 1. The second group will receive remdesivir and baricitinib brand name Olumiant a modulator of inflammation that is approved by FDA to treat certain adult patients with.
 Source: ejcancer.com
Source: ejcancer.com
To treat inpatients with moderate-to-severe COVID-19. The candidate vaccines developed by AstraZeneca and Oxford AZD1222 and the Janssen Pharmaceutical Companies of Johnson Johnson JNJ-7843672 or Ad26COV2S are viral vector-based. Characteristics of Antiviral Agents That Are Approved or Under. Treatments and Vaccines NIH is speeding the development of the most promising vaccines and treatments. In the study more than 6000 patients hospitalized with COVID-19 randomly received either dexamethasone or standard treatment.
 Source: uptodate.com
Source: uptodate.com
153 Anti-SARS-CoV-2 Antibody Products. 3E1 Thyroid 9E1 5E1 Thyroid 2E2 2E-8. The NIH COVID-19 treatment guidelines recommend the use of dexamethasone in certain people hospitalized with severe COVID-19. In a new study a research team led by Dr. 153 Anti-SARS-CoV-2 Antibody Products.
 Source: medicaldevice-network.com
Source: medicaldevice-network.com
We used GRADE to rate the certainty of evidence for the following outcomes 1. Characteristics of Antiviral Agents That Are Approved or Under Evaluation for the Treatment of COVID-19 listed is Ivermectin on NIH website httpswwwcovid19treatmentguidelinesnihgovtablestable-2e 12. Ivermectin is APPROVED by the NIH as a treatment for COVID-19. While a vaccine is a central part of the effort to prevent infection and illness there is no specific antiviral treatment recommended for COVID-19. Total vaccine doses administered per 100 population.
 Source: directorsblog.nih.gov
Source: directorsblog.nih.gov
Secondary outcome was the duration of NIV. The NIH COVID-19 treatment guidelines recommend the use of dexamethasone in certain people hospitalized with severe COVID-19. Some people have sought alternative remedies to prevent or to treat COVID-19. For severe cases treatment should include oxygen supplementation and care to support vital organ functions as needed. The databases Medline Embase and WHO COVID-19 were searched for all publications from 2019 to 9 February 2021 using a combination of the terms COVID 19 SARSCoV2 coronavirus therapy lopinavir ritonavir ribavirin remdesivir hydroxychloroquine corticosteroids anakinra tocilizumab convalescent plasma.
This site is an open community for users to submit their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site helpful, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title nih covid treatment table 2e by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.





