Your Monoclonal treatment for covid images are ready in this website. Monoclonal treatment for covid are a topic that is being searched for and liked by netizens now. You can Find and Download the Monoclonal treatment for covid files here. Get all free photos.
If you’re looking for monoclonal treatment for covid images information related to the monoclonal treatment for covid interest, you have visit the ideal blog. Our site always gives you suggestions for downloading the maximum quality video and picture content, please kindly hunt and locate more enlightening video content and images that fit your interests.
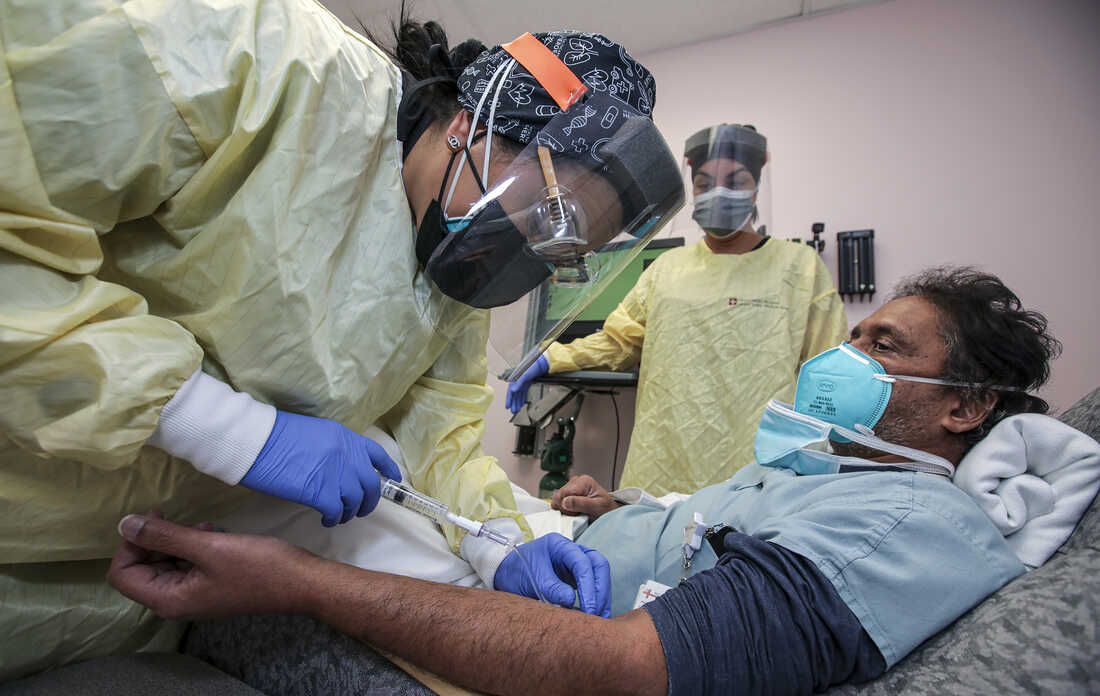
Monoclonal Treatment For Covid. Monoclonal antibody treatments are proving to be a highly effective tool in preventing severe COVID-19 outcomes in at-risk patients such as those who are immunocompromised. Monoclonal Antibody treatments must be recommended by a healthcare professional based on a persons health history and how long theyve had COVID-19 symptoms according to DHEC officials. The successful treatment of an aggressive fatal virus supports the potential of monoclonal antibodies for the treatment of COVID-19. Treatment with COVID-19 monoclonal antibodies is done through a one-time intravenous IV infusion or by subcutaneous injection shot given under the skin.
 Covid 19 Monoclonal Antibody Therapies Patients Stanford Health Care From stanfordhealthcare.org
Covid 19 Monoclonal Antibody Therapies Patients Stanford Health Care From stanfordhealthcare.org
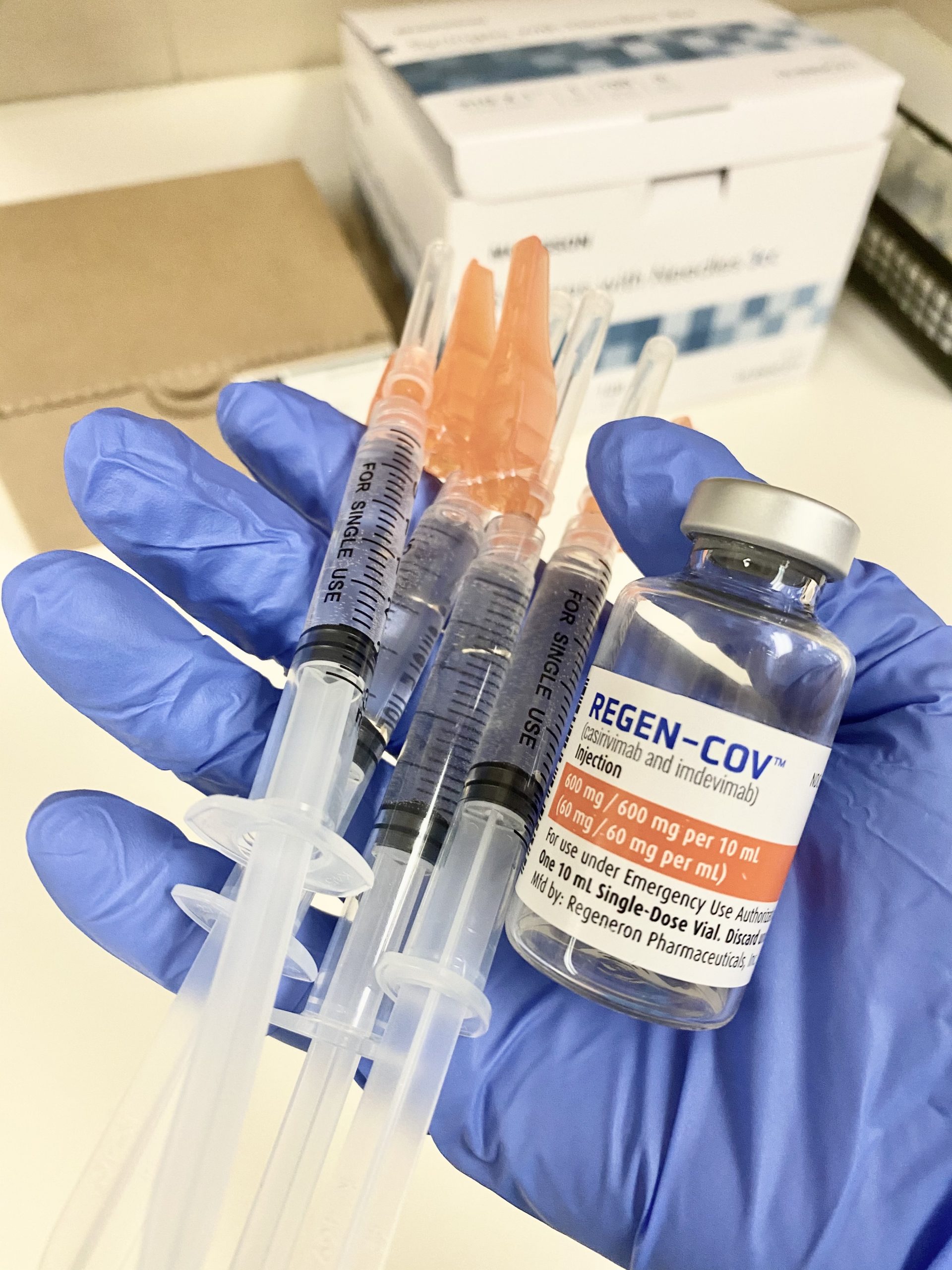
Monoclonal antibody therapy is a way of treating COVID-19 for people who have tested positive have had mild symptoms for seven days or less and are at high risk for developing more serious symptoms. Monoclonal antibodies are a treatment authorized by the US. Monoclonal antibody treatments are proving to be a highly effective tool in preventing severe COVID-19 outcomes in at-risk patients such as those who are immunocompromised. Monoclonal antibodies are used to neutralize the COVID-19 virus and intended to prevent progression of disease. The dosing is the same for both indications casirivimab 600mg and imdevimab 600mg. In the US there are three monoclonal antibody treatments with FDA emergency use authorization for the treatment of COVID-19.
Several SARS-CoV-2 monoclonal antibodies are poised to enter clinical trials during the summer of 2020.
Monoclonal Antibody treatments must be recommended by a healthcare professional based on a persons health history and how long theyve had COVID-19 symptoms according to DHEC officials. Sotrovimab EUA issued May 26 2021 Tocilizumab EUA issued June 24 2021 The FDA authorized the use of these monoclonal antibody therapies to treat mild-to-moderate COVID-19 in adults and pediatric patients when both of these apply. No more than 10 days from symptom onset. Newer research suggests that monoclonal antibody treatment may also help to save lives in a specific subgroup of hospitalized COVID-19 patients. If you have tested positive for COVID-19 you may be able to get monoclonal antibody treatment to help you recover. One of these was a combination of 3 monoclonal antibodies while the other was a single monoclonal antibody.
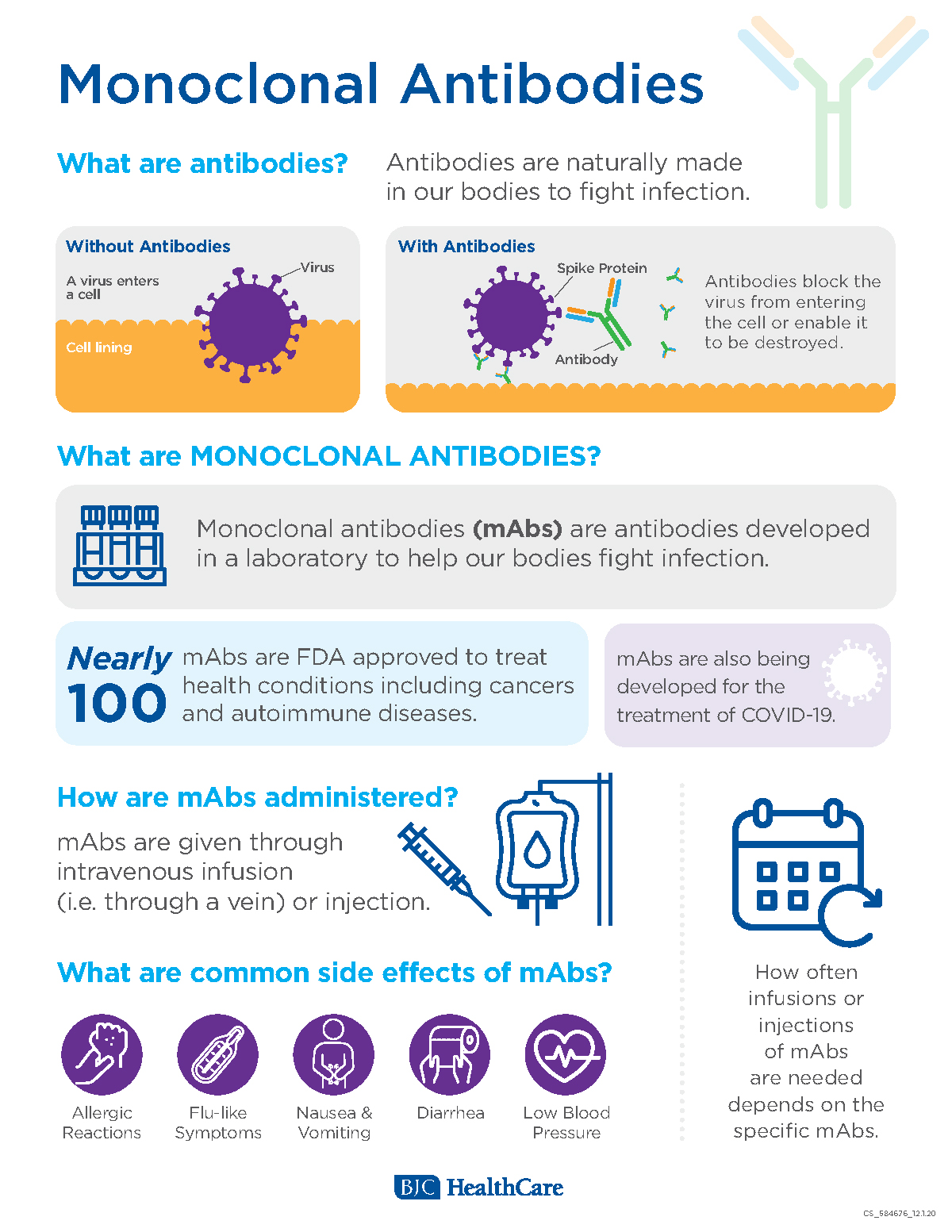 Source: bjc.org
Source: bjc.org
Bamlanivimab plus etesevimab developed by Eli Lilly. The Medicines and Healthcare products Regulatory Agency MHRA has today given approval for the first monoclonal antibody treatment for the. Colorado Department of Public Health and Environment recently launched several new monoclonal antibody treatment buses to help expand access to. This product is not a substitute for vaccination against COVID-19. Treatment with COVID-19 monoclonal antibodies is done through a one-time intravenous IV infusion or by subcutaneous injection shot given under the skin.
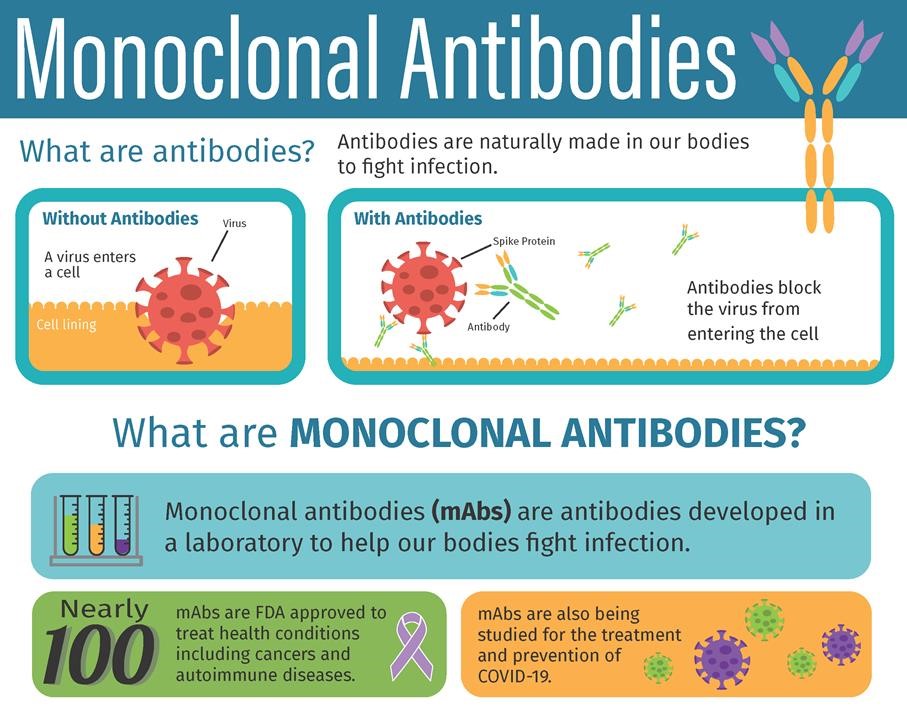 Source: deaconess.com
Source: deaconess.com
Several SARS-CoV-2 monoclonal antibodies are poised to enter clinical trials during the summer of 2020. One of these was a combination of 3 monoclonal antibodies while the other was a single monoclonal antibody. Monoclonal antibody treatments for COVID are antibodies that are created in a lab that are then infused into the body through an IV. Unlike a vaccine that triggers your body to create antibodies. The goal of this therapy is to help prevent hospitalizations reduce viral loads and lessen symptom severity.
 Source: nbcnews.com
Source: nbcnews.com
Monoclonal antibodies are a treatment authorized by the US. Government is currently supplying REGEN-COV casirivimab and imdevimab for the treatment and post-exposure prophylaxis of COVID-19. Bamlanivimab plus etesevimab developed by Eli Lilly. At high risk of developing severe disease. KDVR As Colorado urges people to consider monoclonal antibody treatment to help reduce COVID-19 hospitalizations a Boulder woman shared her.
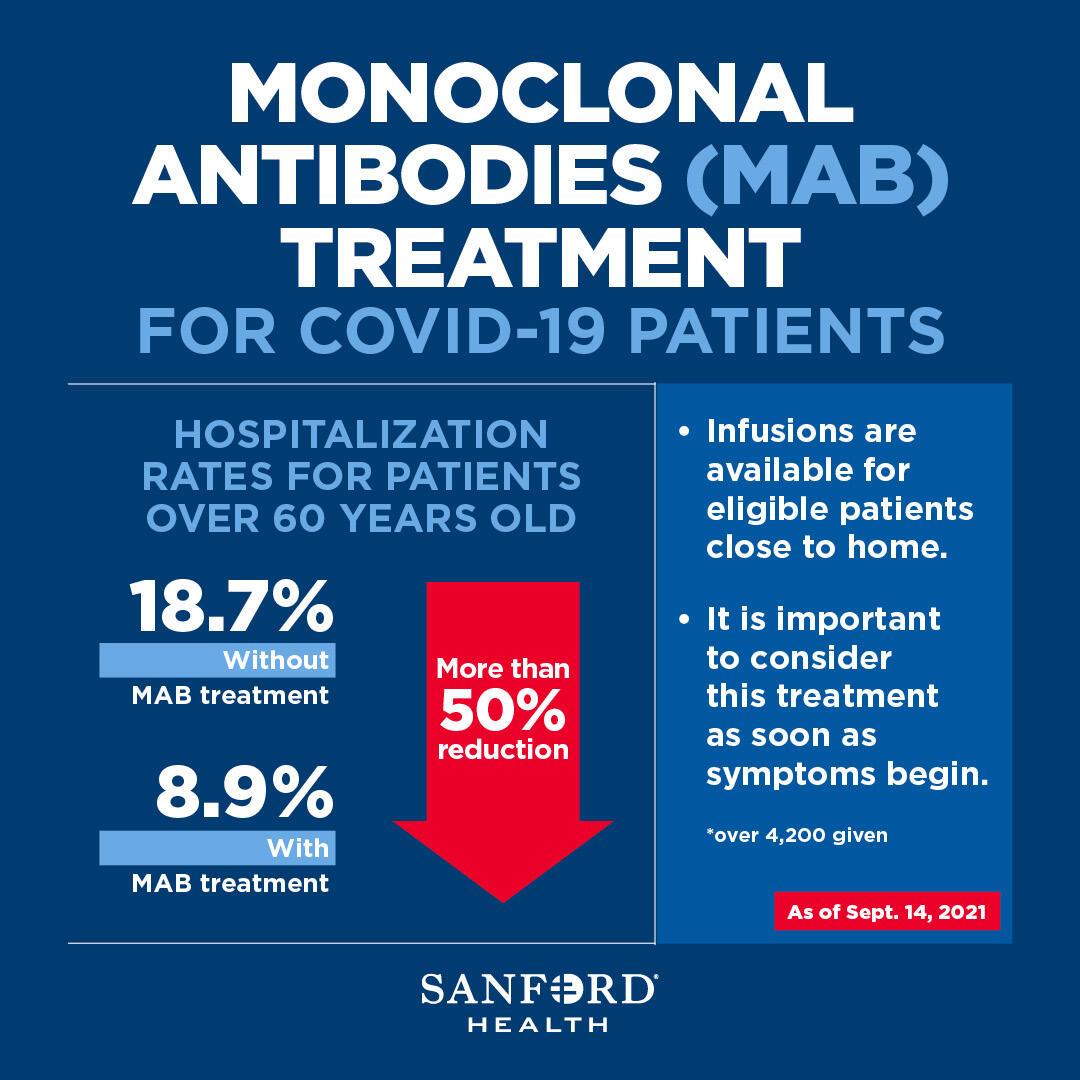 Source: news.sanfordhealth.org
Source: news.sanfordhealth.org
Monoclonal antibodies help the immune system recognize and respond more effectively to the COVID-19 virus. Colorado Department of Public Health and Environment recently launched several new monoclonal antibody treatment buses to help expand access to. Monoclonal antibody treatments for COVID are antibodies that are created in a lab that are then infused into the body through an IV. Today the FDA issued an emergency use authorization for the investigational monoclonal antibody therapy bamlanivimab for the treatment of mild-to-moderate COVID-19 in adult and pediatric patients. Bamlanivimab plus etesevimab developed by Eli Lilly.
 Source: innovativecorona.com
Source: innovativecorona.com
Monoclonal antibodies used for the treatment of COVID-19 target the viral spike protein which prevents viral entry. Several SARS-CoV-2 monoclonal antibodies are poised to enter clinical trials during the summer of 2020. At this time UCHealth uses these products which are available by FDA Emergency Use Authorization. Monoclonal antibodies are a treatment authorized by the US. The goal of this therapy is to help prevent hospitalizations reduce viral loads and lessen symptom severity.
 Source: sciencedirect.com
Source: sciencedirect.com
Monoclonal antibody treatments are proving to be a highly effective tool in preventing severe COVID-19 outcomes in at-risk patients such as those who are immunocompromised. Casirivimab plus imdevimab made by Regeneron Pharmaceuticals. Today the FDA issued an emergency use authorization for the investigational monoclonal antibody therapy bamlanivimab for the treatment of mild-to-moderate COVID-19 in adult and pediatric patients. Sotrovimab EUA issued May 26 2021 Tocilizumab EUA issued June 24 2021 The FDA authorized the use of these monoclonal antibody therapies to treat mild-to-moderate COVID-19 in adults and pediatric patients when both of these apply. Unlike a vaccine that triggers your body to create antibodies.
 Source: healthaffairs.org
Source: healthaffairs.org
If you have tested positive for COVID-19 you may be able to get monoclonal antibody treatment to help you recover. The great hope for drug treatments against Covid-19 the monoclonal antibodies are failing against variants of the virus such as those that have emerged in South Africa and Brazil. Today the FDA issued an emergency use authorization for the investigational monoclonal antibody therapy bamlanivimab for the treatment of mild-to-moderate COVID-19 in adult and pediatric patients. And sotrovimab which is manufactured by GlaxoSmithKline. The successful treatment of an aggressive fatal virus supports the potential of monoclonal antibodies for the treatment of COVID-19.
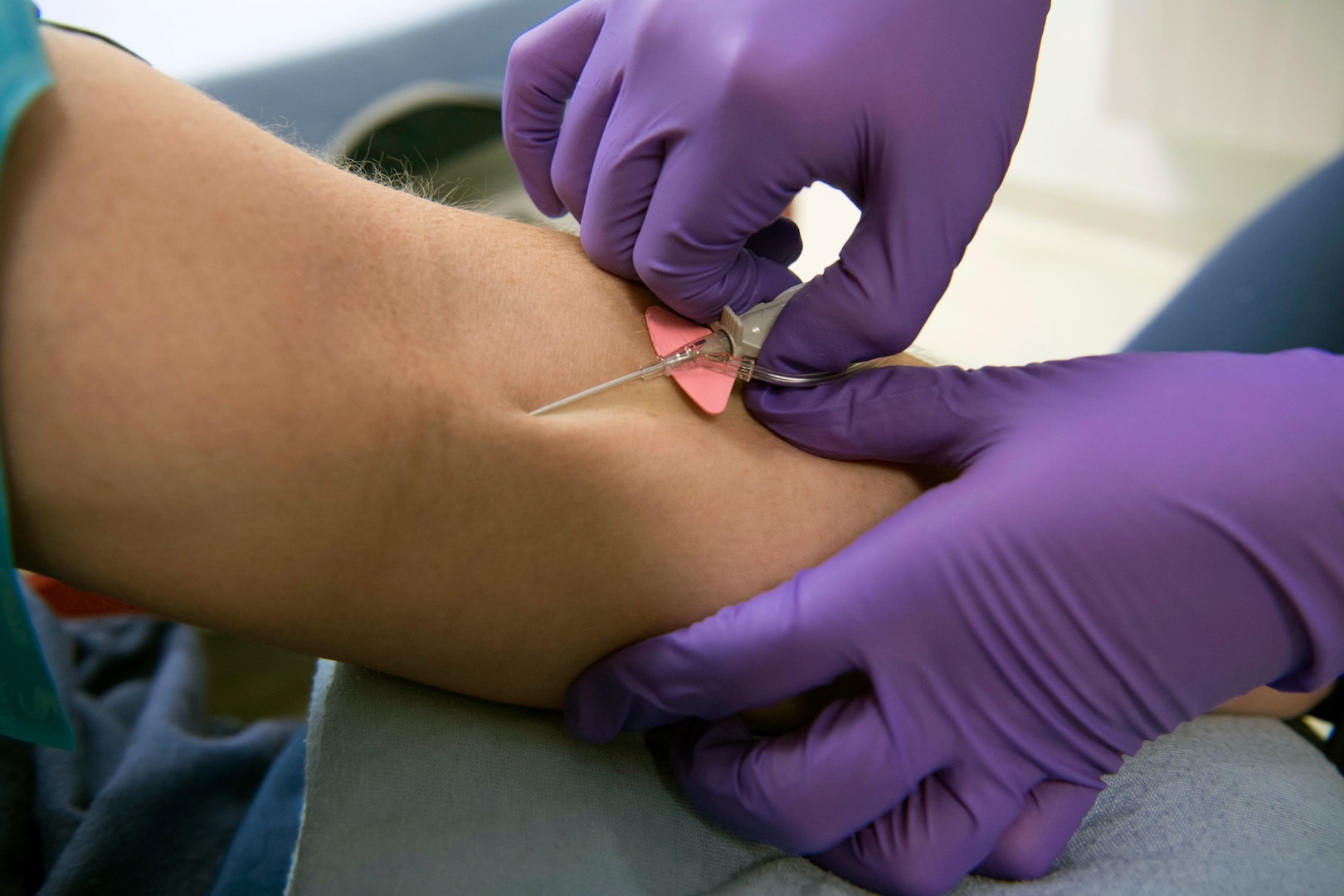
Today the FDA issued an emergency use authorization for the investigational monoclonal antibody therapy bamlanivimab for the treatment of mild-to-moderate COVID-19 in adult and pediatric patients. Monoclonal Antibody treatments must be recommended by a healthcare professional based on a persons health history and how long theyve had COVID-19 symptoms according to DHEC officials. While getting vaccinated is critical this treatment can arm you with one more tool to protect yourself if you happen to get infected with COVID-19. COVID-19 monoclonal antibody therapeutics may be available to patients who test positive for COVID-19 are. Age 12 years and older.
 Source: eehealth.org
Source: eehealth.org
The Medicines and Healthcare products Regulatory Agency MHRA has today given approval for the first monoclonal antibody treatment for the. And sotrovimab which is manufactured by GlaxoSmithKline. Monoclonal antibodies are used to neutralize the COVID-19 virus and intended to prevent progression of disease. The goal of this therapy is to help prevent hospitalizations reduce viral loads and lessen symptom severity. Monoclonal antibodies are manmade versions of the antibodies that our bodies naturally make to fight invaders such as the SARS-CoV-2 virus.
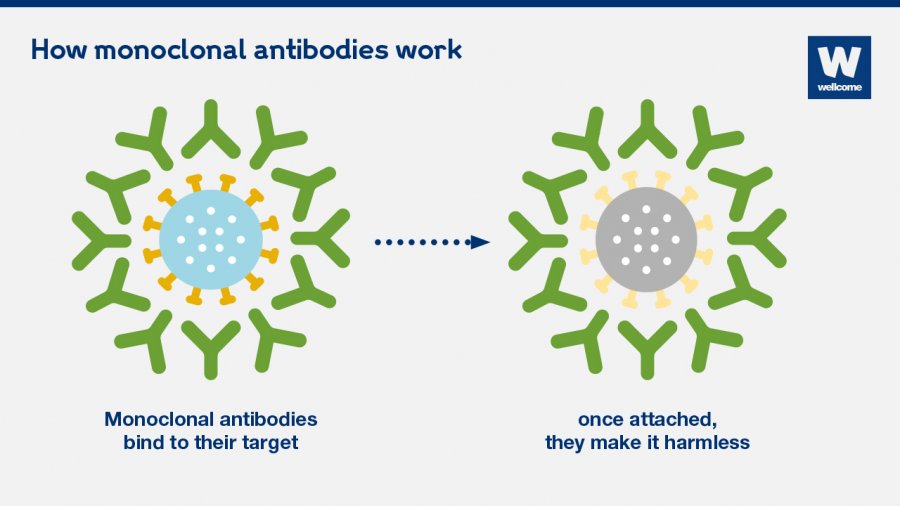 Source: gavi.org
Source: gavi.org
Age 12 years and older. Bamlanivimab plus etesevimab developed by Eli Lilly. Find Monoclonal Antibody Treatment. Monoclonal Antibody treatments must be recommended by a healthcare professional based on a persons health history and how long theyve had COVID-19 symptoms according to DHEC officials. Casirivimab plus imdevimab made by Regeneron Pharmaceuticals.
 Source: healthcare.utah.edu
Source: healthcare.utah.edu
Sotrovimab EUA issued May 26 2021 Tocilizumab EUA issued June 24 2021 The FDA authorized the use of these monoclonal antibody therapies to treat mild-to-moderate COVID-19 in adults and pediatric patients when both of these apply. Colorado Department of Public Health and Environment recently launched several new monoclonal antibody treatment buses to help expand access to. Monoclonal antibody therapy is a way of treating COVID-19 for people who have tested positive have had mild symptoms for seven days or less and are at high risk for developing more serious symptoms. Government is currently supplying REGEN-COV casirivimab and imdevimab for the treatment and post-exposure prophylaxis of COVID-19. The Medicines and Healthcare products Regulatory Agency MHRA has today given approval for the first monoclonal antibody treatment for the.
 Source: news-medical.net
Source: news-medical.net
Monoclonal antibodies used for the treatment of COVID-19 target the viral spike protein which prevents viral entry. Find Monoclonal Antibody Treatment. Casirivimab plus imdevimab made by Regeneron Pharmaceuticals. And sotrovimab which is manufactured by GlaxoSmithKline. Today the FDA issued an emergency use authorization for the investigational monoclonal antibody therapy bamlanivimab for the treatment of mild-to-moderate COVID-19 in adult and pediatric patients.
 Source: nghs.com
Source: nghs.com
At high risk of developing severe disease. The dosing is the same for both indications casirivimab 600mg and imdevimab 600mg. Treatment with COVID-19 monoclonal antibodies is done through a one-time intravenous IV infusion or by subcutaneous injection shot given under the skin. KDVR As Colorado urges people to consider monoclonal antibody treatment to help reduce COVID-19 hospitalizations a Boulder woman shared her. The patient has a positive COVID-19 test result.
 Source: wfla.com
Source: wfla.com
Monoclonal Antibody treatments must be recommended by a healthcare professional based on a persons health history and how long theyve had COVID-19 symptoms according to DHEC officials. Experiencing mild to moderate symptoms. The dosing is the same for both indications casirivimab 600mg and imdevimab 600mg. While getting vaccinated is critical this treatment can arm you with one more tool to protect yourself if you happen to get infected with COVID-19. Monoclonal antibodies help the immune system recognize and respond more effectively to the COVID-19 virus.
 Source: ce.mayo.edu
Source: ce.mayo.edu
Monoclonal antibodies are used to neutralize the COVID-19 virus and intended to prevent progression of disease. Monoclonal antibodies help the immune system recognize and respond more effectively to the COVID-19 virus. While getting vaccinated is critical this treatment can arm you with one more tool to protect yourself if you happen to get infected with COVID-19. Monoclonal antibody treatments are not a. Monoclonal antibodies are a treatment authorized by the US.
 Source: npr.org
Source: npr.org
Monoclonal antibodies are biological drugs used to treat cancers certain types of arthritis lupus MS and IBD. Monoclonal antibodies help the immune system recognize and respond more effectively to the COVID-19 virus. One EUA for a monoclonal antibody product includes use for preventative prophylaxis treatment after being exposed to SARS-CoV-2. The great hope for drug treatments against Covid-19 the monoclonal antibodies are failing against variants of the virus such as those that have emerged in South Africa and Brazil. The Medicines and Healthcare products Regulatory Agency MHRA has today given approval for the first monoclonal antibody treatment for the.
 Source: medpagetoday.com
Source: medpagetoday.com
The successful treatment of an aggressive fatal virus supports the potential of monoclonal antibodies for the treatment of COVID-19. Monoclonal antibody therapy reduces deaths and hospitalizations in non-hospitalized patients with risk factors for severe disease progression. The successful treatment of an aggressive fatal virus supports the potential of monoclonal antibodies for the treatment of COVID-19. Monoclonal antibodies are a treatment authorized by the US. This type of therapy relies on monoclonal antibodies.
 Source: umc.edu
Source: umc.edu
Bamlanivimab plus etesevimab developed by Eli Lilly. One EUA for a monoclonal antibody product includes use for preventative prophylaxis treatment after being exposed to SARS-CoV-2. While getting vaccinated is critical this treatment can arm you with one more tool to protect yourself if you happen to get infected with COVID-19. Monoclonal antibody treatments are not a. Monoclonal antibodies used for the treatment of COVID-19 target the viral spike protein which prevents viral entry.
This site is an open community for users to do sharing their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site serviceableness, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title monoclonal treatment for covid by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.





