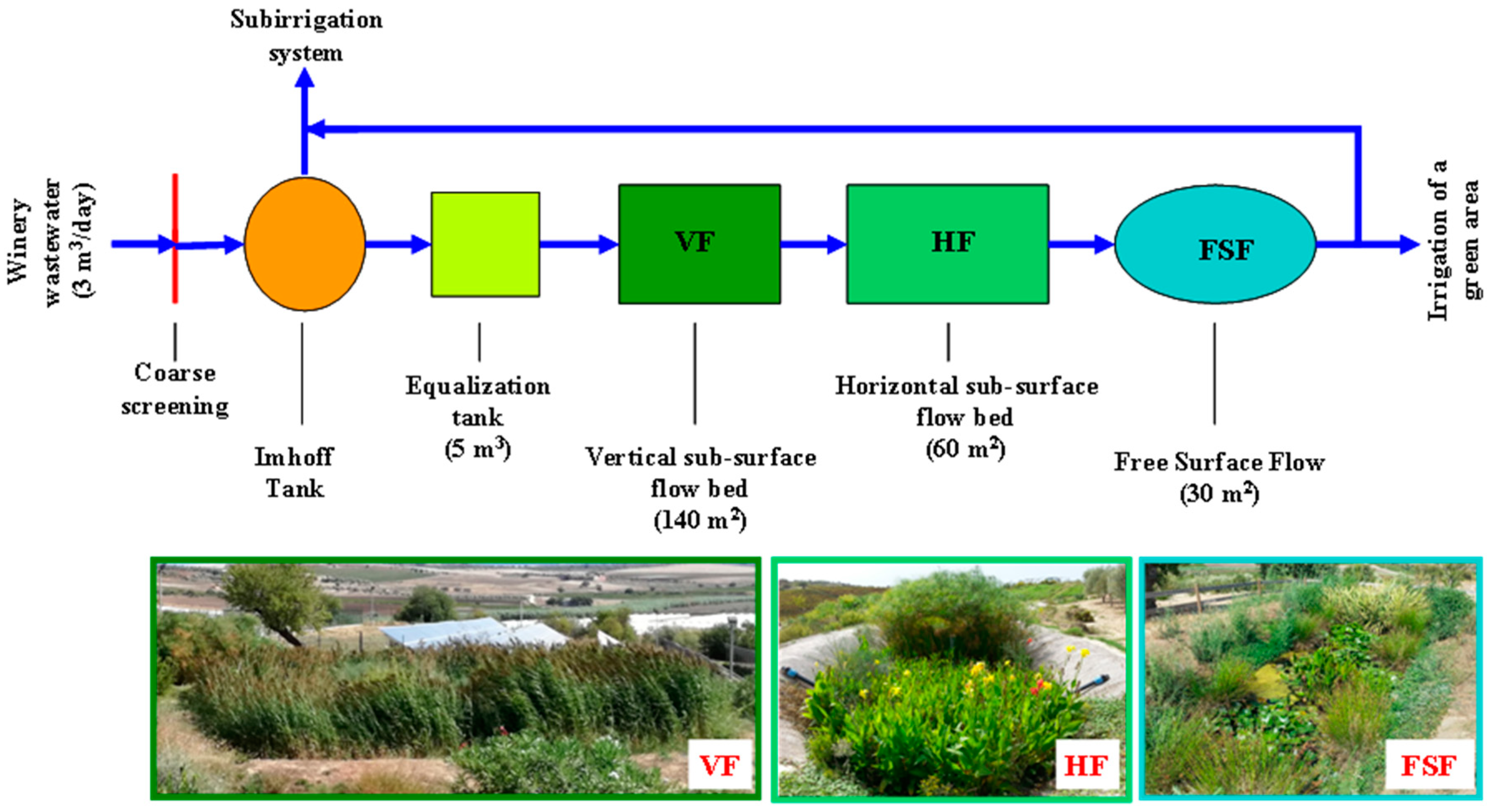
Your Monoclonal antibody treatment indiana images are ready. Monoclonal antibody treatment indiana are a topic that is being searched for and liked by netizens now. You can Get the Monoclonal antibody treatment indiana files here. Find and Download all royalty-free photos and vectors.
If you’re searching for monoclonal antibody treatment indiana pictures information connected with to the monoclonal antibody treatment indiana keyword, you have come to the ideal site. Our website always provides you with suggestions for seeking the highest quality video and image content, please kindly surf and locate more enlightening video content and images that fit your interests.
Monoclonal Antibody Treatment Indiana. Monoclonal antibody therapy is a treatment for people who have tested positive for COVID-19 within 10 days. The therapy has been shown to help high. There is currently no charge for the medication but there is a 550 infusion charge that is covered by most insurances. Standard or custom product.

Ad Reagents devices kits for all protein analysis needs. The medications are bamlanivimab from Eli Lilly and a combination of. Callers can be connected to Crush COVID a support center that provides information about monoclonal antibody treatment on behalf of participating providers. ChanintornvGetty Images Eli Lillys COVID. Mild to moderate confirmed COVID -19 infection. Accelerate cell gene therapy pipelines.
Ad Cell line engineering workflows that assure monoclonality with regulatory-ready reports.
Ad Cell line engineering workflows that assure monoclonality with regulatory-ready reports. The Indiana Department of Healths LTC Medical Director Dr. Ad Cell line engineering workflows that assure monoclonality with regulatory-ready reports. Monoclonal antibodies are laboratory-made proteins that mimic the antibodies the immune system produces when a person gets sick with COVID-19. The therapy has been shown to help high-risk COVID-19 patients avoid hospitalization and recover at home. There are currently two monoclonal antibody treatments that are specifically directed against the spike protein of SARS-CoV-2 designed to block the virus attachment and entry into human cells.
 Source: bioprocessintl.com
Source: bioprocessintl.com
Food and Drug Administraton for outpatient use. Mild to moderate confirmed COVID -19 infection. ChanintornvGetty Images Eli Lillys COVID. Ad Reagents devices kits for all protein analysis needs. Standard or custom product.
 Source: survivorcorps.com
Source: survivorcorps.com
Food and Drug Administration for outpatient use. The therapy has been shown to help high-risk COVID-19 patients avoid hospitalization and recover at home. Monoclonal antibody therapy is a treatment for people who have tested positive for COVID-19 within 10 days. Hoosiers who call 211 will now have another resource available to them. Monoclonal antibody therapy is the first COVID-19 treatment granted emergency use authorization by the US.
 Source: jbc.org
Source: jbc.org
MAB therapy is available under emergency use authorization for those who meet ALL the following criteria. The therapy has been shown to help high-risk COVID-19 patients avoid hospitalization and recover at home. Monoclonal antibody therapy is the first COVID-19 treatment given emergency use authorization by the FDA for outpatient use. Preservation purification clean up quantitation detection. The Indiana Department of Healths LTC Medical Director Dr.
 Source: survivorcorps.com
Source: survivorcorps.com
Monoclonal antibody therapy is the first COVID-19 treatment granted emergency use authorization by the US. The drugs block the. The therapy has been shown to help high-risk COVID-19 patients avoid hospitalization and recover at home. Hoosiers who call 211 will now have another resource available to them. Ad Custom Fully Human Antibody Production 100 GuaranteedHigh AffinityContact Us.
 Source: npr.org
Source: npr.org
Nearly 100 sites across Indiana are serving as infusion centers. Monoclonal antibody therapy MAB can be helpful to prevent clinical deterioration and hospitalization in confirmed COVID-19 cases. Standard or custom product. It has been shown to help high-risk COVID patients avoid hospitalization and recover at home. Monoclonal antibody therapy is the first COVID-19 treatment granted emergency use authorization by the US.
 Source: bioprocessintl.com
Source: bioprocessintl.com
The hotline will help connect people to monoclonal antibody treatment centers. Monoclonal antibody therapy is the first COVID-19 treatment granted emergency use authorization by the US. Monoclonal antibody treatments which are meant to keep people with COVID-19 out of the hospital and prevent people from dying is the first COVID-19 treatment granted emergency use by the US. The hotline will help connect people to monoclonal antibody treatment centers. Monoclonal antibody therapy MAB can be helpful to prevent clinical deterioration and hospitalization in confirmed COVID-19 cases.
 Source: goshennews.com
Source: goshennews.com
Mild to moderate confirmed COVID -19 infection. Beacon Medical Group physicians should call 574-546-8001 to schedule patients for a monoclonal. Accelerate cell gene therapy pipelines. Food and Drug Administration FDA announced it revised the existing emergency use authorization EUA of bamlanivimab and etesevimab to additionally authorize the treatment of mild to moderate COVID-19 in all younger pediatric patients including newborns. Nearly 100 sites across Indiana are serving as infusion centers.
 Source: deaconess.com
Source: deaconess.com
Nearly 100 sites across Indiana are serving as infusion centers. The one-time infusion has been shown to help high-risk COVID-19 patients avoid. Monoclonal antibody therapy is the first COVID-19 treatment granted emergency use authorization by the US. Ad Reagents devices kits for all protein analysis needs. The Indiana Department of Health has launched a new resource for Hoosiers with questions about COVID-19 treatments.
 Source: montanafreepress.org
Source: montanafreepress.org
Hoosiers who call 211 will now have another resource available to them. Vuppalanchi provided a reminder on the distribution and use of monoclonal antibody treatments. Accelerate cell gene therapy pipelines. Hoosiers who call 211 will now have another resource available to them. The hotline will help connect people to monoclonal antibody treatment centers.
 Source: wevv.com
Source: wevv.com
Food and Drug Administration for outpatient use. Ad Custom Fully Human Antibody Production 100 GuaranteedHigh AffinityContact Us. Monoclonal antibody therapy is the first COVID-19 treatment granted for emergency use authorization by the US. MAB therapy is available under emergency use authorization for those who meet ALL the following criteria. Monoclonal antibody therapy is a treatment for people who have tested positive for COVID-19 within 10 days.
 Source: ibj.com
Source: ibj.com
Beacon Medical Group physicians should call 574-546-8001 to schedule patients for a monoclonal. The therapy has been shown to help high-risk COVID-19 patients avoid hospitalization and recover at home. Vuppalanchi provided a reminder on the distribution and use of monoclonal antibody treatments. Saint Joseph Health System is providing COVID-19 Monoclonal Antibody MAB Therapy at both our Mishawaka and Plymouth Hospitals. Monoclonal antibodies are administered during a two-hour IV infusion help prevent the coronavirus from invading cells once a person has been infected.
 Source: survivorcorps.com
Source: survivorcorps.com
Monoclonal antibody therapy is the first COVID-19 treatment granted emergency use authorization by the US. Food and Drug Administration for outpatient use. It has been shown to help high-risk COVID patients avoid hospitalization and recover at home. The drugs block the. MAB in treatment of confirmed COVID-19.
 Source: stemcell.com
Source: stemcell.com
Lilly and Regenerons monoclonal antibody treatments for COVID-19 are delivered by infusion to non-hospitalized patients creating unique challenges. Ad Custom Fully Human Antibody Production 100 GuaranteedHigh AffinityContact Us. Monoclonal antibody therapy is the first COVID-19 treatment granted emergency use authorization by the US. Monoclonal antibody therapy is the first COVID-19 treatment granted for emergency use authorization by the US. Callers can be connected to Crush COVID a support center that provides information about monoclonal antibody treatment on behalf of participating providers.

There are currently two monoclonal antibody treatments that are specifically directed against the spike protein of SARS-CoV-2 designed to block the virus attachment and entry into human cells. Nearly 100 sites across Indiana are serving as infusion centers. Department of Health and Human Services HHS. Saint Joseph Health System is providing COVID-19 Monoclonal Antibody MAB Therapy at both our Mishawaka and Plymouth Hospitals. The revised EUA was issued to Indiana-based Eli Lilly Co.
 Source: scientificamerican.com
Source: scientificamerican.com
Monoclonal antibodies are administered during a two-hour IV infusion help prevent the coronavirus from invading cells once a person has been infected. Please see below for Dr. The Indiana Department of Health has launched a new resource for Hoosiers with questions about COVID-19 treatments. The therapy has been shown to help high-risk COVID-19 patients avoid hospitalization and recover at home. Monoclonal antibody therapy is the first COVID-19 treatment given emergency use authorization by the FDA for outpatient use.
 Source: reidhealth.org
Source: reidhealth.org
Monoclonal antibody therapy is a treatment for people who have tested positive for COVID-19 within 10 days. Food and Drug. Ad Cell line engineering workflows that assure monoclonality with regulatory-ready reports. Accelerate cell gene therapy pipelines. Mild to moderate confirmed COVID -19 infection.
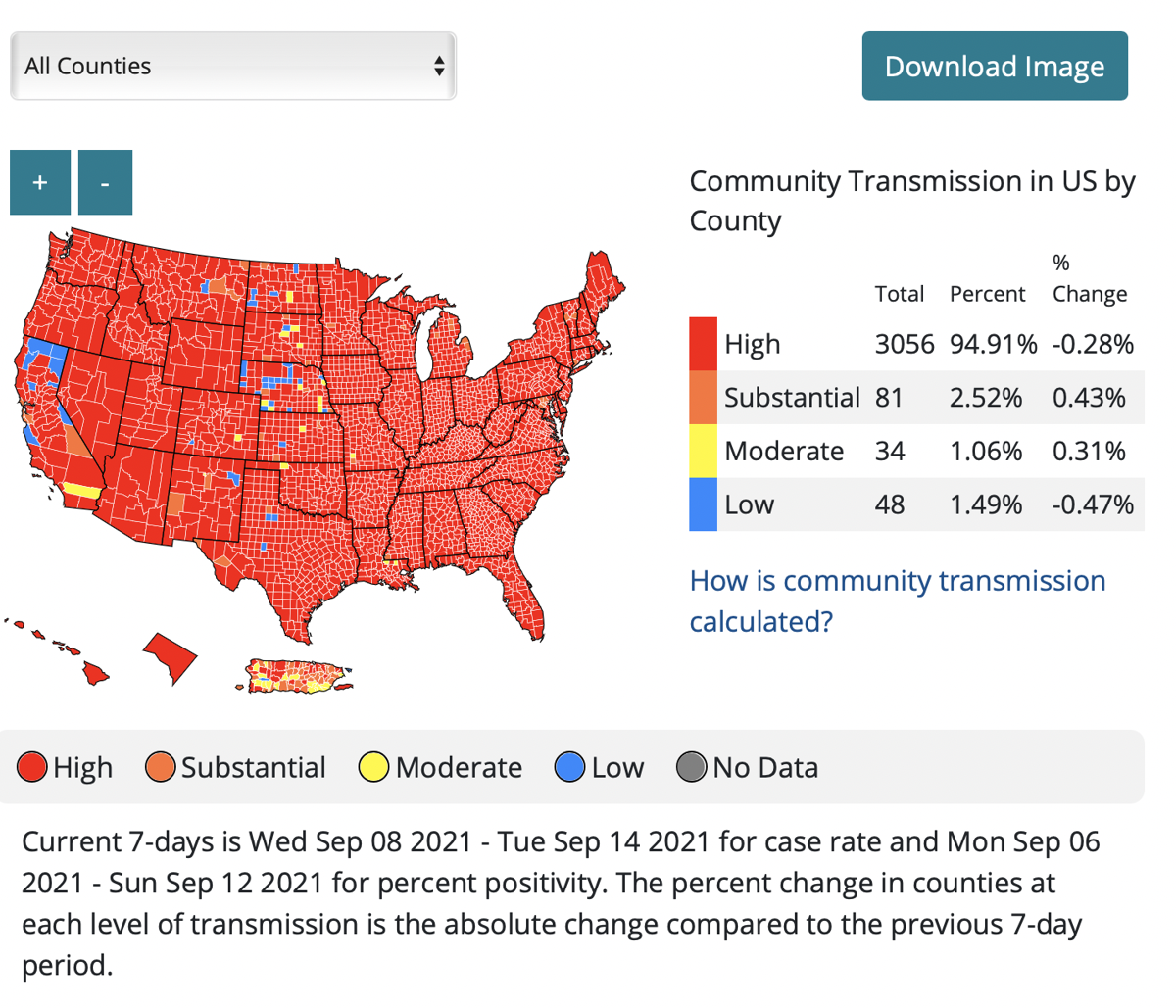 Source: willistonherald.com
Source: willistonherald.com
Monoclonal antibody therapyis a treatment for. There is currently no charge for the medication but there is a 550 infusion charge that is covered by most insurances. Food and Drug Administration for outpatient use. The Indiana Department of Health has launched a new resource for Hoosiers with questions about COVID-19 treatments. Ad Cell line engineering workflows that assure monoclonality with regulatory-ready reports.
 Source: wfyi.org
Source: wfyi.org
MAB in treatment of confirmed COVID-19. The medications are bamlanivimab from Eli Lilly and a combination of. MAB in treatment of confirmed COVID-19. Monoclonal antibody therapy is the first COVID-19 treatment granted emergency use authorization by the US. Food and Drug Administration for outpatient use.
This site is an open community for users to share their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site convienient, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title monoclonal antibody treatment indiana by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.