Your Monoclonal antibody treatment for covid 19 images are ready. Monoclonal antibody treatment for covid 19 are a topic that is being searched for and liked by netizens now. You can Find and Download the Monoclonal antibody treatment for covid 19 files here. Find and Download all royalty-free vectors.
If you’re looking for monoclonal antibody treatment for covid 19 pictures information connected with to the monoclonal antibody treatment for covid 19 keyword, you have visit the right site. Our website frequently provides you with hints for seeing the maximum quality video and picture content, please kindly surf and locate more enlightening video content and images that match your interests.
Monoclonal Antibody Treatment For Covid 19. The FDA has issued EUAs for a number of investigational monoclonal antibodies that. If the monoclonal antibodies are given relatively soon in. UPMC received two monoclonal antibody infusion treatment products. Treatment with COVID-19 monoclonal antibodies is done through a one-time intravenous IV infusion or by subcutaneous injection shot given under the skin.
 Logan Health Continues Innovative Covid 19 Monoclonal Antibody Treatment From logan.org
Logan Health Continues Innovative Covid 19 Monoclonal Antibody Treatment From logan.org
Monoclonal antibody therapy has been suggested as an option in preventing progression to severe COVID-19 infection in high-risk individuals and reducing hospitalizations. Treatment works best when you. If you have tested positive for COVID-19 you may be able to get monoclonal antibody treatment to help you recover. Monoclonal antibody therapy can prevent severe illness hospitalization and death in high-risk patients who have contracted or been exposed to COVID-19. Clinical trials show that Regenerons monoclonal antibody treatment a combination of two antibodies called casirivimab and imdevimab reduces COVID-19-related hospitalization or deaths in high. Monoclonal antibody treatment can be used in people 12 years of age and older who weigh at least 88 pounds 40 kg who are at high risk for severe COVID-19 including hospitalization or death for.
Monoclonal antibodies are used to neutralize the COVID-19 virus and intended to prevent progression of disease.
In 2020 the FDA authorized several different monoclonal antibodies to treat COVID-19. No more than 10 days from symptom onset. The dosing is the same for both indications casirivimab 600mg and imdevimab 600mg. UPMC received two monoclonal antibody infusion treatment products. Monoclonal antibody therapy can prevent severe illness hospitalization and death in high-risk patients who have contracted or been exposed to COVID-19. The use of anti-SARS-CoV-2 monoclonal antibodies can be considered in pregnant people with COVID-19 especially in those who have additional risk factors for severe disease.
 Source: healthpolicy.duke.edu
Source: healthpolicy.duke.edu
Government is currently supplying REGEN-COV casirivimab and imdevimab for the treatment and post-exposure prophylaxis of COVID-19. Monoclonal antibody treatment can be used in people 12 years of age and older who weigh at least 88 pounds 40 kg who are at high risk for severe COVID-19 including hospitalization or death for. This activity outlines the indications actions contraindications and adverse events for monoclonal antibody therapy as a valuable treatment for outpatient COVID-19 infections. This page provides a broad overview of therapies that are currently recommended for COVID-19 treatment. The use of anti-SARS-CoV-2 monoclonal antibodies can be considered in pregnant people with COVID-19 especially in those who have additional risk factors for severe disease.
 Source: cleveland.com
Source: cleveland.com
One of these treatments is sotrovimab while the other is a combination of the drugs casirivimab and imdevimab. An antibody is a protein that is naturally produced by the immune system in response to an infection. The Medicines and Healthcare products Regulatory Agency MHRA has today given approval for the first monoclonal antibody treatment. COVID-19 monoclonal antibody therapeutics may be available to patients who test positive for COVID-19 are. UPMC received two monoclonal antibody infusion treatment products.
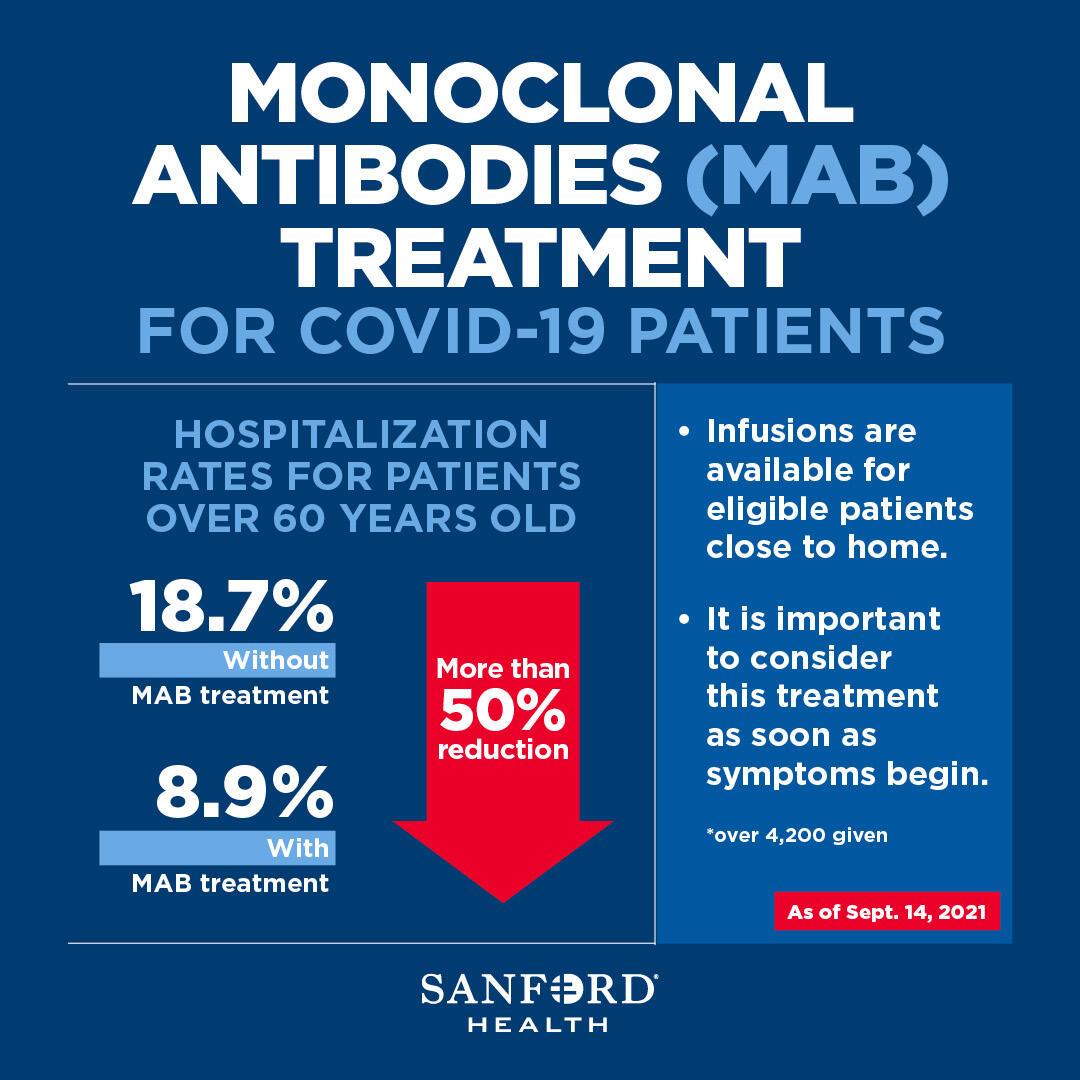 Source: news.sanfordhealth.org
Source: news.sanfordhealth.org
One of these treatments is sotrovimab while the other is a combination of the drugs casirivimab and imdevimab. Monoclonal antibodies are used to neutralize the COVID-19 virus and intended to prevent progression of disease. Authorised by Greg Hunt MP Liberal Party of Australia Somerville Victoria. This treatment can help keep you from getting seriously sick and keep you out of the hospital. Monoclonal Antibody Therapy for COVID-19.
 Source: sciencedirect.com
Source: sciencedirect.com
Authorised by Greg Hunt MP Liberal Party of Australia Somerville Victoria. A potential limitation of monoclonal antibodies for treatment of COVID-19 is the unknown bioavailability of passively infused IgG in tissues affected by the disease especially the lungs which serve as a key target of SARS-CoV-2 infection. Monoclonal antibody therapy can prevent severe illness hospitalization and death in high-risk patients who have contracted or been exposed to COVID-19. COVID-19 monoclonal antibody therapeutics may be available to patients who test positive for COVID-19 are. At this time UCHealth uses these products which are available by FDA Emergency Use Authorization.
 Source: ce.mayo.edu
Source: ce.mayo.edu
The dosing is the same for both indications casirivimab 600mg and imdevimab 600mg. Government is currently supplying REGEN-COV casirivimab and imdevimab for the treatment and post-exposure prophylaxis of COVID-19. The FDA has issued EUAs for a number of investigational monoclonal antibodies that. The use of anti-SARS-CoV-2 monoclonal antibodies can be considered in pregnant people with COVID-19 especially in those who have additional risk factors for severe disease. Monoclonal antibodies are a new treatment for outpatients with COVID-19 who are at risk of progression to severe disease.
 Source: logan.org
Source: logan.org
This treatment can help keep you from getting seriously sick and keep you out of the hospital. Monoclonal antibodies are a new treatment for outpatients with COVID-19 who are at risk of progression to severe disease. Treatment is free and vaccination status does not matter. Manufactured by GSK sotrovimab will be the first COVID-19 monoclonal antibody treatment available for use in this country with the complete treatment requiring just one dose administered via IV infusion in a healthcare facility. Monoclonal antibody therapy is a way of treating COVID-19 for people who have tested positive have had mild symptoms for seven days or less and are at high risk for developing more serious symptoms.
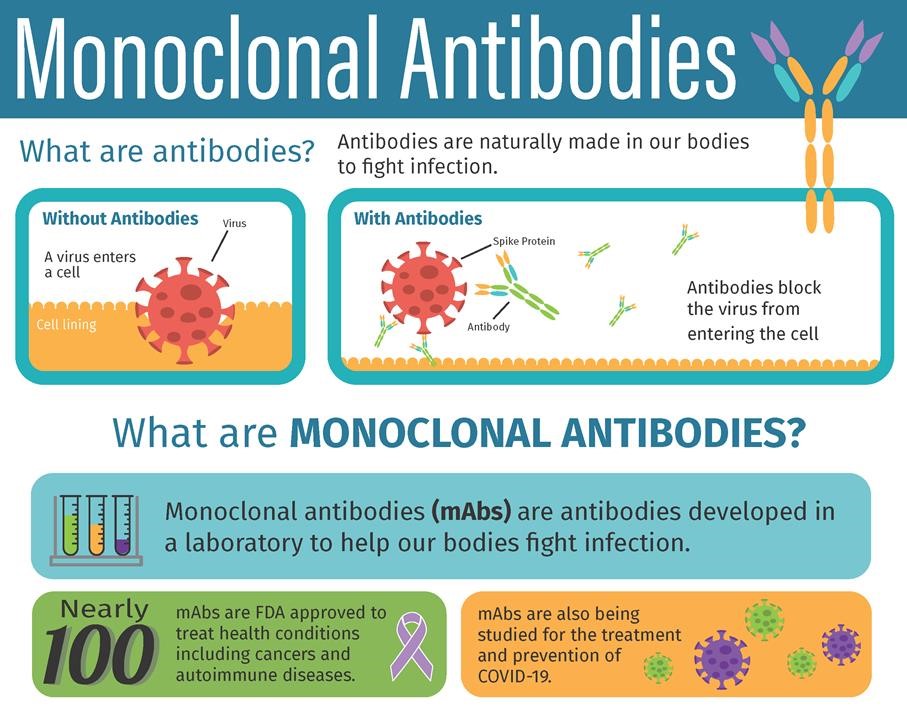 Source: deaconess.com
Source: deaconess.com
Monoclonal antibody therapy is a way of treating COVID-19 for people who have tested positive have had mild symptoms for seven days or less and are at high risk for developing more serious symptoms. These treatments are widely available in Florida. Treatment of mild to moderate symptoms of COVID-19. The antibodies work to block the virus that causes COVID-19 from attaching to human cells making it more difficult for the virus to reproduce and cause harm. A potential limitation of monoclonal antibodies for treatment of COVID-19 is the unknown bioavailability of passively infused IgG in tissues affected by the disease especially the lungs which serve as a key target of SARS-CoV-2 infection.
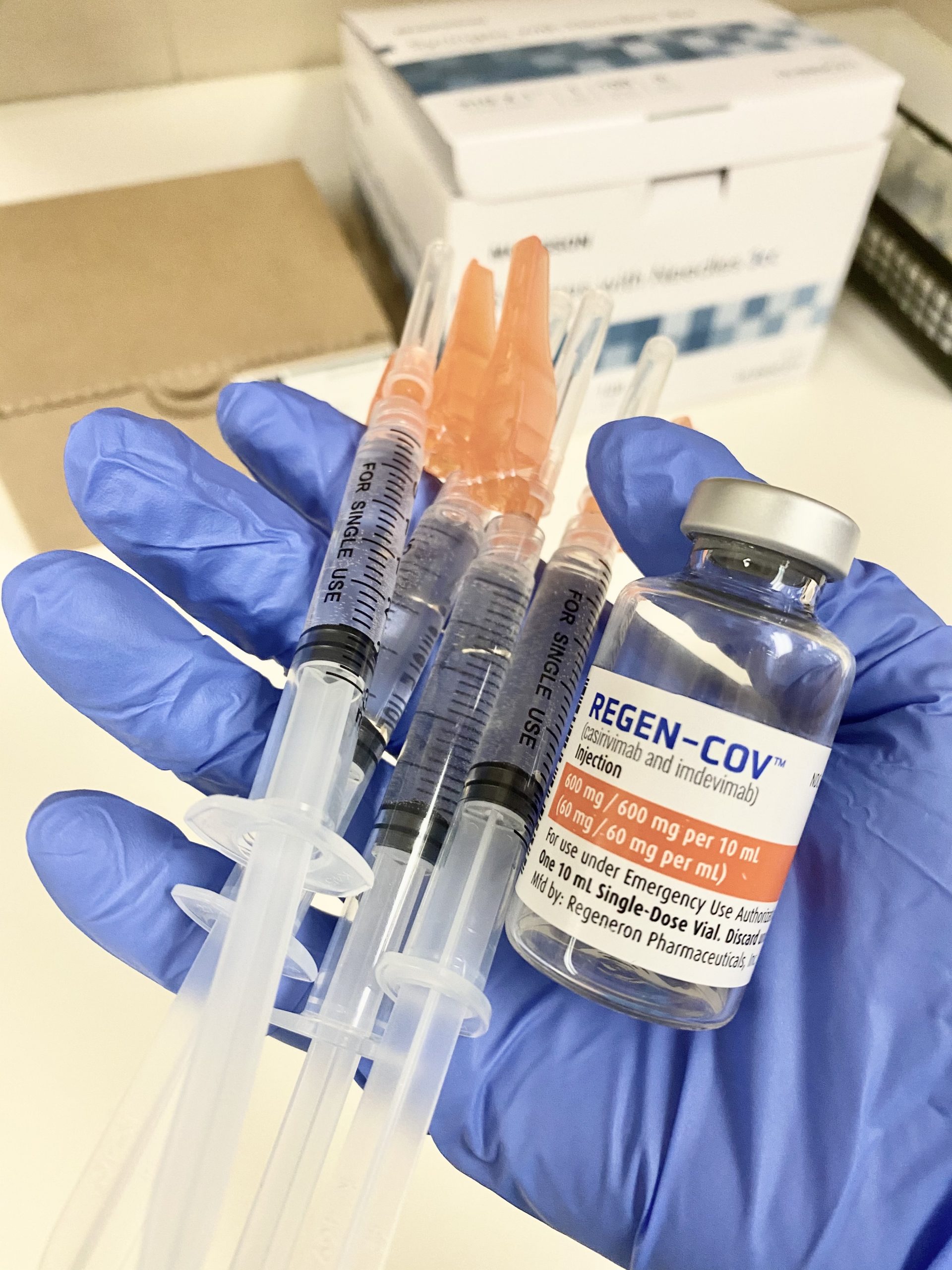 Source: nghs.com
Source: nghs.com
Monoclonal antibody treatment is generally given within 10 days of a positive COVID-19 test. Monoclonal antibody treatment is generally given within 10 days of a positive COVID-19 test. One of these treatments is sotrovimab while the other is a combination of the drugs casirivimab and imdevimab. Monoclonal antibody treatments are not a substitute for vaccination against COVID-19. An antibody is a protein that is naturally produced by the immune system in response to an infection.
 Source: stanfordhealthcare.org
Source: stanfordhealthcare.org
Clinical trials show that Regenerons monoclonal antibody treatment a combination of two antibodies called casirivimab and imdevimab reduces COVID-19-related hospitalization or deaths in high. The Medicines and Healthcare products Regulatory Agency MHRA has today given approval for the first monoclonal antibody treatment. Today the FDA issued an emergency use authorization for the investigational monoclonal antibody therapy bamlanivimab for the treatment of mild-to-moderate COVID-19 in adult and pediatric patients. If you have tested positive for COVID-19 you may be able to get monoclonal antibody treatment to help you recover. The FDA has issued EUAs for a number of investigational monoclonal antibodies that.
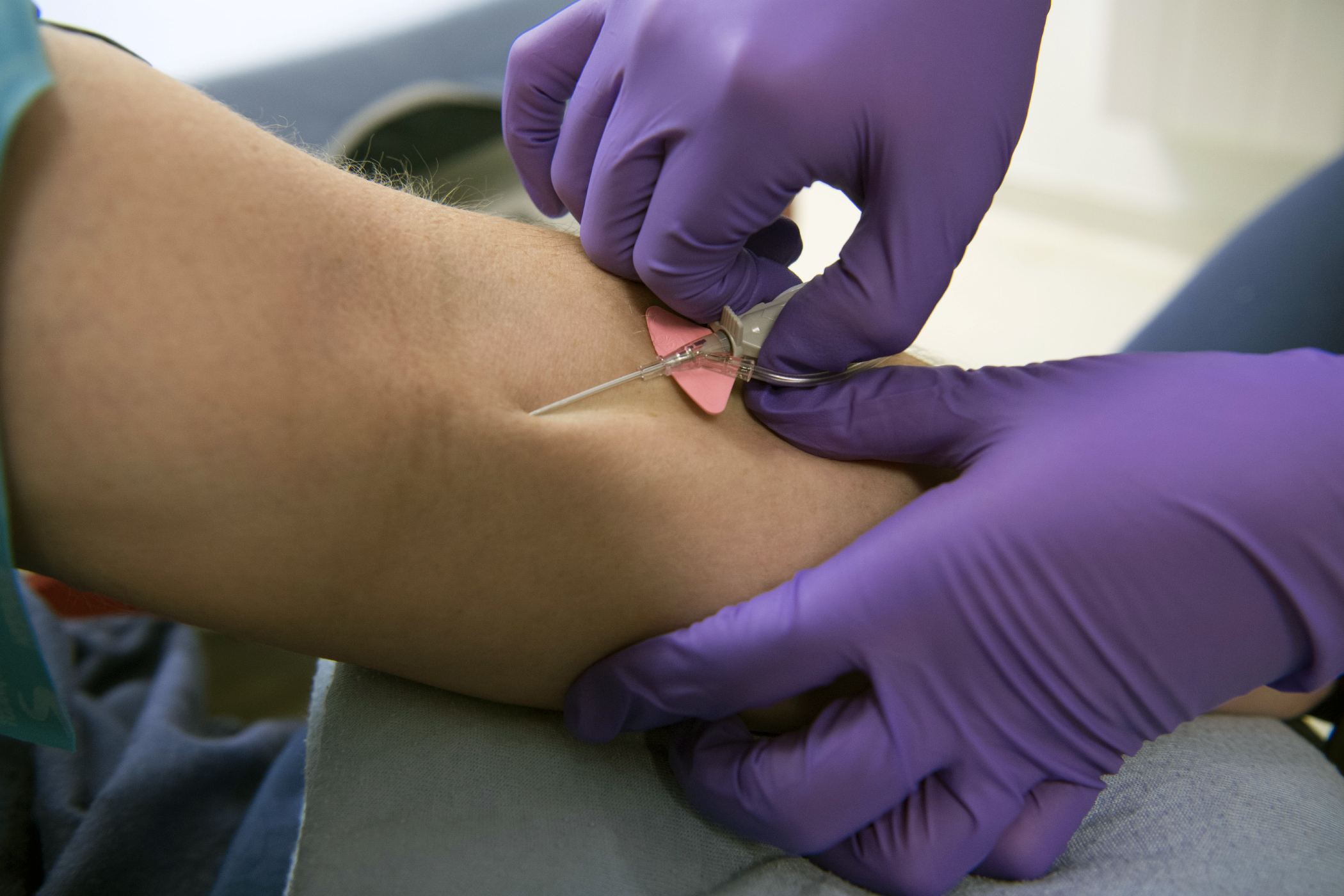
Monoclonal antibody treatment is generally given within 10 days of a positive COVID-19 test. The Medicines and Healthcare products Regulatory Agency MHRA has today given approval for the first monoclonal antibody treatment. 9 2020 the FDA issued an EUA for a single infusion of 700 mg bamlanivimab for the treatment of mild-to-moderate COVID-19 in adult and certain pediatric patients. To be eligible patients must. If the monoclonal antibodies are given relatively soon in.
 Source: post-gazette.com
Source: post-gazette.com
COVID-19 monoclonal antibody therapeutics may be available to patients who test positive for COVID-19 are. Experiencing mild to moderate symptoms. Authorised by Greg Hunt MP Liberal Party of Australia Somerville Victoria. This section of the COVID-19 Treatment Guidelines complements that guidance. One of these treatments is sotrovimab while the other is a combination of the drugs casirivimab and imdevimab.
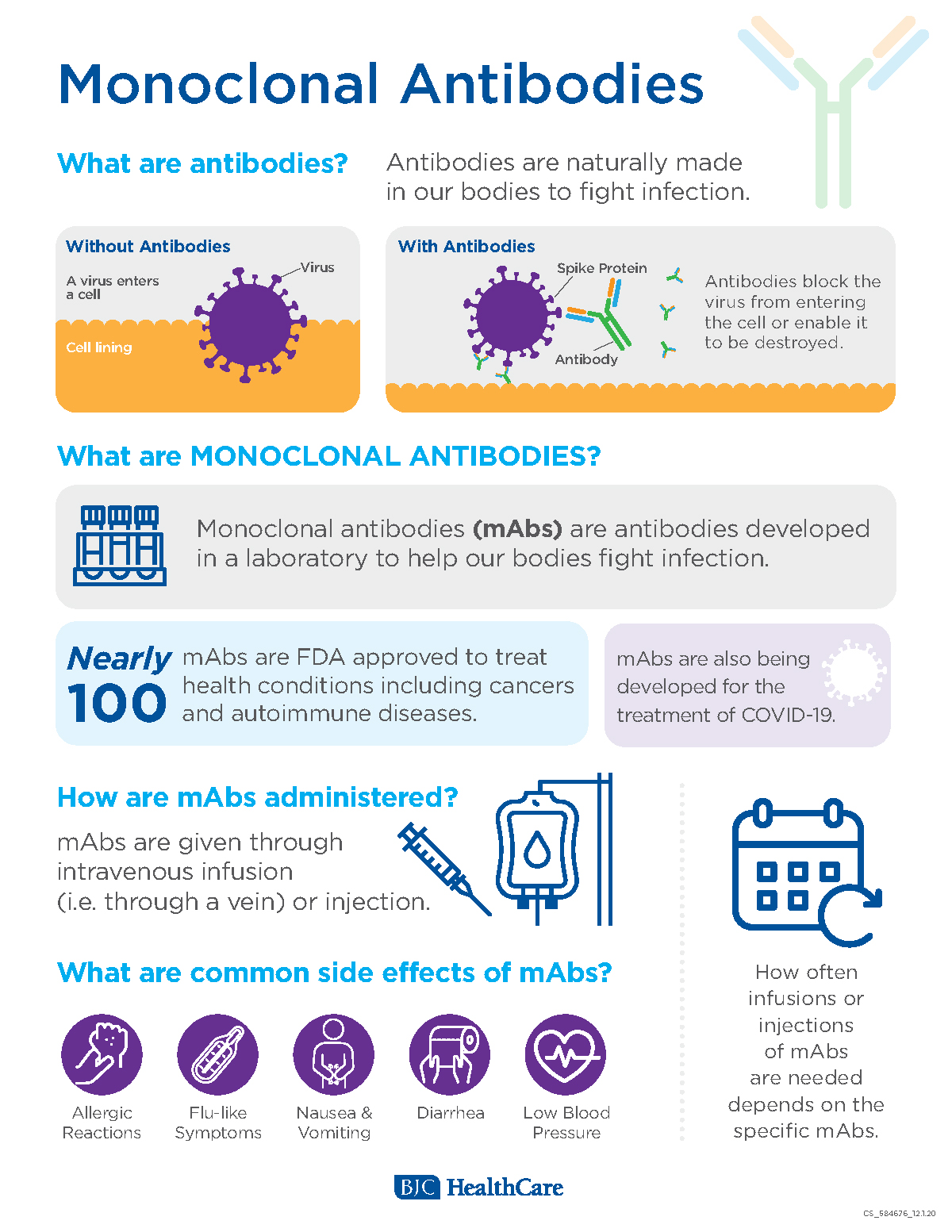 Source: bjc.org
Source: bjc.org
Manufactured by GSK sotrovimab will be the first COVID-19 monoclonal antibody treatment available for use in this country with the complete treatment requiring just one dose administered via IV infusion in a healthcare facility. Monoclonal antibodies to fight COVID-19 are artificially manufactured antibodies designed to mimic your bodys natural antibodies. The use of anti-SARS-CoV-2 monoclonal antibodies can be considered in pregnant people with COVID-19 especially in those who have additional risk factors for severe disease. Experiencing mild to moderate symptoms. Monoclonal Antibody Treatment for COVID-19 Monoclonal antibody treatment is for people who have COVID-19 or were recently exposed to someone who has COVID-19 and are not hospitalized.
 Source: healthaffairs.org
Source: healthaffairs.org
The dosing is the same for both indications casirivimab 600mg and imdevimab 600mg. This treatment can help keep you from getting seriously sick and keep you out of the hospital. The FDA can issue emergency use authorizations EUAs. At this time UCHealth uses these products which are available by FDA Emergency Use Authorization. Treatment of mild to moderate symptoms of COVID-19.
 Source: creakyjoints.org
Source: creakyjoints.org
The FDA can issue emergency use authorizations EUAs. Monoclonal antibody therapy has been suggested as an option in preventing progression to severe COVID-19 infection in high-risk individuals and reducing hospitalizations. Monoclonal antibody treatments are not a substitute for vaccination against COVID-19. Today the FDA issued an emergency use authorization for the investigational monoclonal antibody therapy bamlanivimab for the treatment of mild-to-moderate COVID-19 in adult and pediatric patients. The dosing is the same for both indications casirivimab 600mg and imdevimab 600mg.
 Source: wfla.com
Source: wfla.com
Treatment with COVID-19 monoclonal antibodies is done through a one-time intravenous IV infusion or by subcutaneous injection shot given under the skin. Clinical trials show that Regenerons monoclonal antibody treatment a combination of two antibodies called casirivimab and imdevimab reduces COVID-19-related hospitalization or deaths in high. Treatment with COVID-19 monoclonal antibodies is done through a one-time intravenous IV infusion or by subcutaneous injection shot given under the skin. 9 2020 the FDA issued an EUA for a single infusion of 700 mg bamlanivimab for the treatment of mild-to-moderate COVID-19 in adult and certain pediatric patients. Monoclonal antibody therapy can prevent severe illness hospitalization and death in high-risk patients who have contracted or been exposed to COVID-19.
 Source: bloomberg.com
Source: bloomberg.com
One of these treatments is sotrovimab while the other is a combination of the drugs casirivimab and imdevimab. Monoclonal Antibody Treatment for COVID-19 Monoclonal antibody treatment is for people who have COVID-19 or were recently exposed to someone who has COVID-19 and are not hospitalized. Treatment is free and vaccination status does not matter. Monoclonal antibody treatment is generally given within 10 days of a positive COVID-19 test. Monoclonal antibody therapy has been suggested as an option in preventing progression to severe COVID-19 infection in high-risk individuals and reducing hospitalizations.
 Source: nbcnews.com
Source: nbcnews.com
Monoclonal antibodies to fight COVID-19 are artificially manufactured antibodies designed to mimic your bodys natural antibodies. This page provides a broad overview of therapies that are currently recommended for COVID-19 treatment. The FDA can issue emergency use authorizations EUAs. Monoclonal antibodies are a new treatment for outpatients with COVID-19 who are at risk of progression to severe disease. These treatments are widely available in Florida.
 Source: verywellhealth.com
Source: verywellhealth.com
Monoclonal antibody treatments are not a substitute for vaccination against COVID-19. Manufactured by GSK sotrovimab will be the first COVID-19 monoclonal antibody treatment available for use in this country with the complete treatment requiring just one dose administered via IV infusion in a healthcare facility. No more than 10 days from symptom onset. The dosing is the same for both indications casirivimab 600mg and imdevimab 600mg. In 2020 the FDA authorized several different monoclonal antibodies to treat COVID-19.
This site is an open community for users to share their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site convienient, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title monoclonal antibody treatment for covid 19 by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.