Your Bam treatment for covid 19 images are available in this site. Bam treatment for covid 19 are a topic that is being searched for and liked by netizens now. You can Download the Bam treatment for covid 19 files here. Get all free photos and vectors.
If you’re searching for bam treatment for covid 19 pictures information linked to the bam treatment for covid 19 keyword, you have come to the right blog. Our website always provides you with hints for viewing the highest quality video and picture content, please kindly hunt and locate more informative video content and images that fit your interests.
Bam Treatment For Covid 19. While the safety and effectiveness of this investigational therapy continues to be evaluated bamlanivimab was shown in clinical trials to reduce COVID-19-related hospitalization or emergency. Yes but you must wait until 90 days have passed since you received your monoclonal antibody infusion. It does not contain any COVID-19 virus or parts and does not come from another person who has had COVID. However his doctors at Our Lady of the Lake OLOL decided to put him on The Bam Its an antibody transfusion treatment for high-risk COVID-19 patients.
 Bamlanivimab Ly Cov555 For The Treatment Of Covid 19 Usa From clinicaltrialsarena.com
Bamlanivimab Ly Cov555 For The Treatment Of Covid 19 Usa From clinicaltrialsarena.com
In light of in vitro studies showing that these monoclonal antibodies were ineffective against the Beta B1351. Bamlanivimab or BAM is approved for high risk adult and pediatric COVID-19 positive patients with mild to moderate symptoms. Bamlanivimab is a neutralizing antibody drug. FDA Revokes Emergency Use Authorization for Monoclonal Antibody Bamlanivimab Alternative monoclonal antibody therapies authorized to treat patients with. The laboratory-made antibody mimics a naturally occurring one which is known to fight. However his doctors at Our Lady of the Lake OLOL decided to put him on The Bam Its an antibody transfusion treatment for high-risk COVID-19 patients.
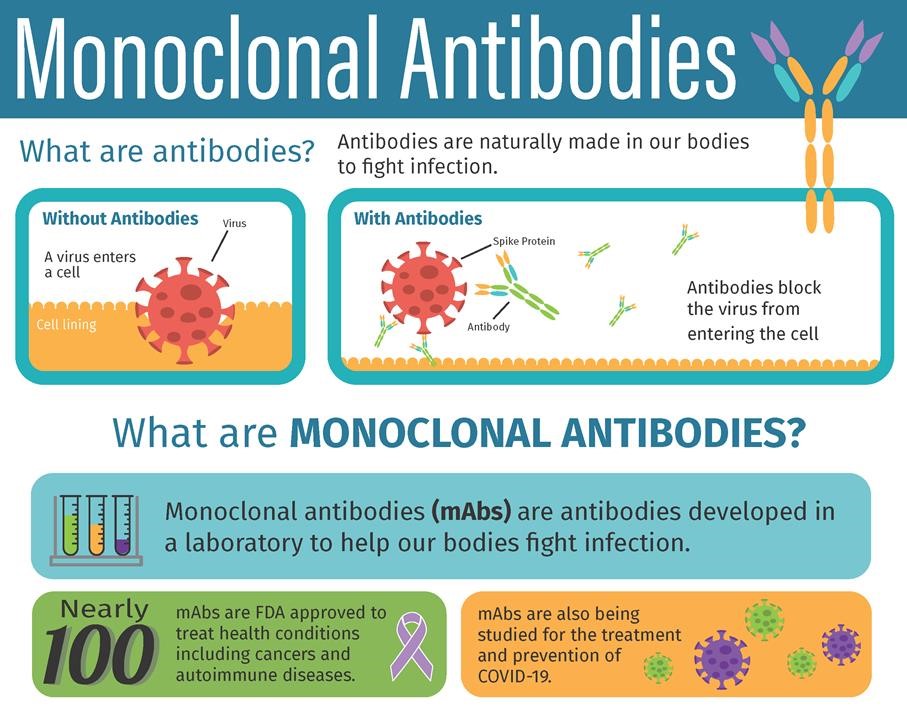
It contains man-made antibodies similar to the antibodies of people who have recovered from COVID.
That includes those 65 or over and those with certain chronic medical conditions. FDA EUA Indication 2 for outpatient mAb use 2. So far we have treated more than 1000 patients with COVID-19 at high risk for going into the hospital. Some people may have unwanted reactions to bamlanivimab. That includes those 65 or over and those with certain chronic medical conditions. These are called side effects.
 Source: mercyone.org
Source: mercyone.org
In light of in vitro studies showing that these monoclonal antibodies were ineffective against the Beta B1351. However his doctors at Our Lady of the Lake OLOL decided to put him on The Bam Its an antibody transfusion treatment for high-risk COVID-19 patients. Bamlanivimab and etesevimab are investigational medicines used together in adults and adolescents 12 years of age or older who weigh at least 88 pounds 40 kg who are at high risk for developing severe COVID-19 including hospitalization or death for. These treatments are allowed by the US. During this shared decision-making process the patient and the clinical team should consider the safety of the medication for the pregnant or lactating individual and the fetus and the severity of.
 Source: creakyjoints.org
Source: creakyjoints.org
Yes but you must wait until 90 days have passed since you received your monoclonal antibody infusion. FDA EUA Indication 2 for outpatient mAb use 2. In an effort to prevent COVID-19 hospital admissions OSF HealthCare is now offering a cutting edge monoclonal immunotherapy infusion. REGEN-COV can be given IV or SC no preference of one over the other or BamEte only by IV infusion Eligibility Patients 12 years of age and older who are at high risk for progression to severe COVID-19 AND Not fully vaccinated or who are not expected to mount an adequate immune. Some people may have unwanted reactions to bamlanivimab.

FDA EUA Indication 2 for outpatient mAb use 2. These treatments are allowed by the US. Regeneron monoclonal antibody replacing bamlanivimab to keep COVID-19 patients out of the hospital. FDA EUA Indication 2 for outpatient mAb use 2. Under the terms of the EUAs casirivimab and imdevimab administered together and bamlanivimab and etesevimab administered together are authorized in adult and pediatric individuals for treatment of COVID-19 and post-exposure prophylaxis for certain individuals who have been exposed to COVID-19 positive persons.
 Source: bioworld.com
Source: bioworld.com
What side effects can occur. In clinical trials bamlanivimab was effective for the treatment of mild to moderate COVID-19 in adults and pediatric patients with positive results of direct SARS-CoV-2 viral testing There is no adequate approved and available alternative to the emergency use of bamlanivimab for the treatment of Covid. TREATMENT FAQ What is BamlanivimabEtesevimab Infusion Treatment. Under the terms of the EUAs casirivimab and imdevimab administered together and bamlanivimab and etesevimab administered together are authorized in adult and pediatric individuals for treatment of COVID-19 and post-exposure prophylaxis for certain individuals who have been exposed to COVID-19 positive persons. Yes but you must wait until 90 days have passed since you received your monoclonal antibody infusion.

What side effects can occur. Some people may have unwanted reactions to bamlanivimab. In an effort to prevent COVID-19 hospital admissions OSF HealthCare is now offering a cutting edge monoclonal immunotherapy infusion. It contains man-made antibodies similar to the antibodies of people who have recovered from COVID. It contains man-made antibodies similar to the antibodies of people who have recovered from COVID.
 Source: thedacarecovid19.org
Source: thedacarecovid19.org
These are called side effects. Bamlanivimab a popular COVID-19 treatment is proving to be less effective against the delta variant and physicians at Norton Healthcare have responded by shifting to Regeneron treating nearly 100 patients daily. The FDA approval limits bamlanivimab to those at high risk of getting seriously ill or needing hospitalization due to COVID-19. BamlanivimabEtesevimab is a neutralizing antibody drug. In an effort to prevent COVID-19 hospital admissions OSF HealthCare is now offering a cutting edge monoclonal immunotherapy infusion.
 Source: clinicaltrialsarena.com
Source: clinicaltrialsarena.com
TREATMENT FAQ What is Bamlanivimab Infusion Treatment. Bamlanivimab is a neutralizing antibody drug. With the Delta B16172 variant now the predominant variant the EUA was subsequently. It contains man-made antibodies similar to the antibodies of people who have recovered from COVID. REGEN-COV can be given IV or SC no preference of one over the other or BamEte only by IV infusion Eligibility Patients 12 years of age and older who are at high risk for progression to severe COVID-19 AND Not fully vaccinated or who are not expected to mount an adequate immune.
 Source: mercyone.org
Source: mercyone.org
Bamlanivimab or BAM is approved for high risk adult and pediatric COVID-19 positive patients with mild to moderate symptoms. Under the terms of the EUA casirivimab and imdevimab administered together are authorized in adult and pediatric individuals for treatment of COVID-19 and post-exposure prophylaxis for certain individuals who have been exposed to COVID-19 positive persons. Bamlanivimab and etesevimab are investigational medicines used together in adults and adolescents 12 years of age or older who weigh at least 88 pounds 40 kg who are at high risk for developing severe COVID-19 including hospitalization or death for. POSTEXPOSURE PROPHYLAXIS against COVID-19. Yes but you must wait until 90 days have passed since you received your monoclonal antibody infusion.
 Source: wjhl.com
Source: wjhl.com
These are called side effects. What side effects can occur. In clinical trials bamlanivimab was effective for the treatment of mild to moderate COVID-19 in adults and pediatric patients with positive results of direct SARS-CoV-2 viral testing There is no adequate approved and available alternative to the emergency use of bamlanivimab for the treatment of Covid. Bamlanivimab is authorized for the treatment of mild to moderate COVID-19 in adults and pediatric patients with positive results of direct SARS-CoV-2 viral testing who are 12 years of age and older weighing at least 40 kg about 88 pounds and who are at high risk for progressing to severe. Patient Education After Bamlanivimab - 2 - Can I still get the COVID-19 vaccine.
 Source: covid19treatmentguidelines.nih.gov
Source: covid19treatmentguidelines.nih.gov
Under the terms of the EUA casirivimab and imdevimab administered together are authorized in adult and pediatric individuals for treatment of COVID-19 and post-exposure prophylaxis for certain individuals who have been exposed to COVID-19 positive persons. Of those only about 4 have needed to go into the hospital. In clinical trials bamlanivimab was effective for the treatment of mild to moderate COVID-19 in adults and pediatric patients with positive results of direct SARS-CoV-2 viral testing There is no adequate approved and available alternative to the emergency use of bamlanivimab for the treatment of Covid. Bamlanivimab or BAM and casirivimab imdevimab were recently approved by the FDA and are the first of what is likely to be multiple outpatient non-vaccine treatments. Food and Drug Administration FDA under an Emergency Use Authorization EUA while clinical studies continue to look at their usefulness and safety.
 Source: hher24.com
Source: hher24.com
COVID-19 treatment and research information from the US federal government. FDA Revokes Emergency Use Authorization for Monoclonal Antibody Bamlanivimab Alternative monoclonal antibody therapies authorized to treat patients with. In an effort to prevent COVID-19 hospital admissions OSF HealthCare is now offering a cutting edge monoclonal immunotherapy infusion. POSTEXPOSURE PROPHYLAXIS against COVID-19. This compares about to about 9 of such patients who have not had COVID-19 monoclonal antibody treatment.
 Source: forbes.com
Source: forbes.com
And Gamma P1 variants the EUA was revoked in June 2021. Bamlanivimab is authorized for the treatment of mild to moderate COVID-19 in adults and pediatric patients with positive results of direct SARS-CoV-2 viral testing who are 12 years of age and older weighing at least 40 kg about 88 pounds and who are at high risk for progressing to severe. BamlanivimabEtesevimab is a neutralizing antibody drug. Bamlanivimab a popular COVID-19 treatment is proving to be less effective against the delta variant and physicians at Norton Healthcare have responded by shifting to Regeneron treating nearly 100 patients daily. These are called side effects.
 Source: clinicaltrialsarena.com
Source: clinicaltrialsarena.com
Bamlanivimab is a neutralizing antibody drug. In an effort to prevent COVID-19 hospital admissions OSF HealthCare is now offering a cutting edge monoclonal immunotherapy infusion. It contains man-made antibodies similar to the antibodies of people who have recovered from COVID. Monoclonal antibody treatment is a neutralizing antibody medicine meaning it contains man-made antibodies that are like the antibodies of patients who have recovered from COVID-19. That includes those 65 or over and those with certain chronic medical conditions.
 Source: npr.org
Source: npr.org
Pregnant or lactating patients with COVID-19 and their clinical teams should discuss the use of investigational drugs or drugs that are approved for other indications as treatments for COVID-19. It contains man-made antibodies similar to the antibodies of people who have recovered from COVID. In an effort to prevent COVID-19 hospital admissions OSF HealthCare is now offering a cutting edge monoclonal immunotherapy infusion. TREATMENT FAQ What is BamlanivimabEtesevimab Infusion Treatment. In clinical trials bamlanivimab was effective for the treatment of mild to moderate COVID-19 in adults and pediatric patients with positive results of direct SARS-CoV-2 viral testing There is no adequate approved and available alternative to the emergency use of bamlanivimab for the treatment of Covid.
 Source: clinicaltrialsarena.com
Source: clinicaltrialsarena.com
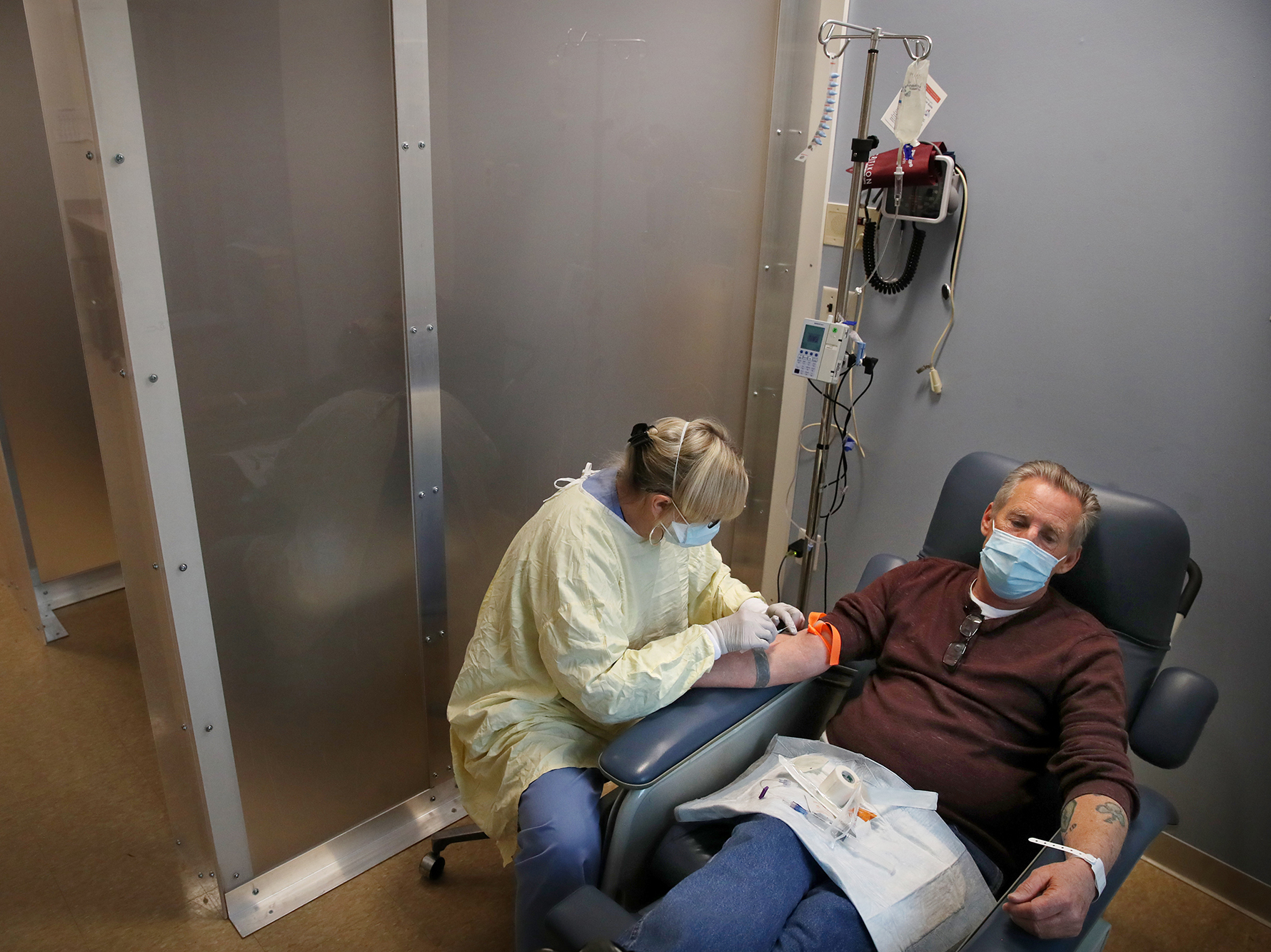
How is Bamlanivimab given. This compares about to about 9 of such patients who have not had COVID-19 monoclonal antibody treatment. COVID-19 treatment and research information from the US federal government. So the BAM infusion with these man-made antibodies reduces the amount of COVID-19 virus thats in your body. Regeneron monoclonal antibody replacing bamlanivimab to keep COVID-19 patients out of the hospital.
 Source: deaconess.com
Source: deaconess.com
Of those only about 4 have needed to go into the hospital. POSTEXPOSURE PROPHYLAXIS against COVID-19. TREATMENT FAQ What is Bamlanivimab Infusion Treatment. Of those only about 4 have needed to go into the hospital. COVID-19 treatment and research information from the US federal government.
 Source: npr.org
Source: npr.org
Patient Education After Bamlanivimab - 2 - Can I still get the COVID-19 vaccine. With the Delta B16172 variant now the predominant variant the EUA was subsequently. Bamlanivimab BAM was previously authorized for the treatment of patients with COVID-19. Under the terms of the EUA casirivimab and imdevimab administered together are authorized in adult and pediatric individuals for treatment of COVID-19 and post-exposure prophylaxis for certain individuals who have been exposed to COVID-19 positive persons. It contains man-made antibodies similar to the antibodies of people who have recovered from COVID.
 Source: carle.org
Source: carle.org
Bamlanivimab or BAM is approved for high risk adult and pediatric COVID-19 positive patients with mild to moderate symptoms. BamlanivimabEtesevimab is a neutralizing antibody drug. What side effects can occur. This compares about to about 9 of such patients who have not had COVID-19 monoclonal antibody treatment. So the BAM infusion with these man-made antibodies reduces the amount of COVID-19 virus thats in your body.
This site is an open community for users to share their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site adventageous, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title bam treatment for covid 19 by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.





